Efficacy of Cilostazol Administration in Alzheimer's Disease Patients with White Matter Lesions: A Positron-Emission Tomography Study
- PMID: 30761509
- PMCID: PMC6554387
- DOI: 10.1007/s13311-018-00708-x
Efficacy of Cilostazol Administration in Alzheimer's Disease Patients with White Matter Lesions: A Positron-Emission Tomography Study
Abstract
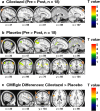
This study tested the efficacy of the phosphodiesterase type III inhibitor cilostazol in Alzheimer's disease patients with white matter lesions treated with donepezil in comparison with donepezil monotherapy using fluorodeoxyglucose (18F) positron-emission tomography (FDG PET). A 24-week, randomized, double-blind, placebo-controlled, parallel-group study was conducted. Thirty-six Alzheimer's disease patients with white matter lesions who received donepezil (n = 18 each in the cilostazol and placebo groups) were enrolled. Participants underwent pre and post FDG PET imaging scans and three rounds of clinical and neuropsychological tests. The cilostazol group did not show a significant decrease of regional glucose metabolism; however, regional glucose metabolism was significantly decreased in the parietal and frontal lobes of the placebo group. The repeated measures ANOVA measuring differences in uptake change revealed that regional glucose metabolism in the left inferior frontal gyrus was significantly more preserved in the cilostazol group than that in the placebo group (p < 0.005). Mean changes from baseline on the Mini-Mental State Exam, Alzheimer's Disease Assessment Scale-cognitive subscale, Alzheimer's Disease Cooperative Study-Activities of Daily Living Inventory, and the Clinical Dementia Rating Sum of Boxes did not differ between the two groups. In the cilostazol group, the increase of glucose metabolism correlated with the improvment of the Alzheimer's Disease Assessment Scale-cognitive score. We conclude that cilostazol treatment added to donepezil may delay the decline in regional cerebral metabolism in Alzheimer's disease with white matter lesions compared with donepezil monotherapy. In additon, our results verified the efficacy of cilostazol in improving or protecting cognitive function in Alzheimer's disease through increased glucose metabolism. However, the long-term effect of cilostazol on cognitive function and Alzheimer's disease modification must be tested in further studies with larger sample size and longer study period. Trial registration: http://clinicaltrials.gov : NCT01409564.
Keywords: Alzheimer’s disease; cilostazol; positron-emission tomography study.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Riluzole, a glutamate modulator, slows cerebral glucose metabolism decline in patients with Alzheimer's disease.Brain. 2021 Dec 31;144(12):3742-3755. doi: 10.1093/brain/awab222. Brain. 2021. PMID: 34145880 Free PMC article. Clinical Trial.
-
Longitudinal PET Evaluation of Cerebral Metabolic Decline in Dementia: A Potential Outcome Measure in Alzheimer's Disease Treatment Studies.Am J Psychiatry. 2002 May;159(5):738-45. doi: 10.1176/appi.ajp.159.5.738. Am J Psychiatry. 2002. PMID: 11986126
-
Evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer's disease: study protocol for a randomised controlled trial (ELAD study).Trials. 2019 Apr 3;20(1):191. doi: 10.1186/s13063-019-3259-x. Trials. 2019. PMID: 30944040 Free PMC article. Clinical Trial.
-
Effects of cilostazol on cognitive function and dementia risk: A systematic review and meta-analysis.Medicine (Baltimore). 2024 Dec 13;103(50):e40668. doi: 10.1097/MD.0000000000040668. Medicine (Baltimore). 2024. PMID: 39686486 Free PMC article.
-
Evidence to Consider Angiotensin II Receptor Blockers for the Treatment of Early Alzheimer's Disease.Cell Mol Neurobiol. 2016 Mar;36(2):259-79. doi: 10.1007/s10571-015-0327-y. Epub 2016 Mar 18. Cell Mol Neurobiol. 2016. PMID: 26993513 Free PMC article. Review.
Cited by
-
Glucose metabolic crosstalk and regulation in brain function and diseases.Prog Neurobiol. 2021 Sep;204:102089. doi: 10.1016/j.pneurobio.2021.102089. Epub 2021 Jun 10. Prog Neurobiol. 2021. PMID: 34118354 Free PMC article. Review.
-
Role of Phosphodiesterase in the Biology and Pathology of Diabetes.Int J Mol Sci. 2020 Nov 3;21(21):8244. doi: 10.3390/ijms21218244. Int J Mol Sci. 2020. PMID: 33153226 Free PMC article. Review.
-
Effect of Resveratrol Combined with Donepezil Hydrochloride on Inflammatory Factor Level and Cognitive Function Level of Patients with Alzheimer's Disease.J Healthc Eng. 2022 Mar 25;2022:9148650. doi: 10.1155/2022/9148650. eCollection 2022. J Healthc Eng. 2022. Retraction in: J Healthc Eng. 2023 Jan 25;2023:9834320. doi: 10.1155/2023/9834320. PMID: 35368930 Free PMC article. Retracted. Clinical Trial.
-
The Role of Sirtuin 1 (SIRT1) in Neurodegeneration.Cureus. 2023 Jun 15;15(6):e40463. doi: 10.7759/cureus.40463. eCollection 2023 Jun. Cureus. 2023. PMID: 37456463 Free PMC article. Review.
-
The anti-platelet drug cilostazol enhances heart rate and interrenal steroidogenesis and exerts a scant effect on innate immune responses in zebrafish.PLoS One. 2023 Oct 30;18(10):e0292858. doi: 10.1371/journal.pone.0292858. eCollection 2023. PLoS One. 2023. PMID: 37903128 Free PMC article.
References
-
- Lee JH, Park SY, Shin YW, Hong KW, Kim CD, Sung SM, et al. Neuroprotection by cilostazol, a phosphodiesterase type 3 inhibitor, against apoptotic white matter changes in rat after chronic cerebral hypoperfusion. Brain research. 2006;1082(1):182–91. doi: 10.1016/j.brainres.2006.01.088. - DOI - PubMed
-
- Patil CS, Singh VP, Kulkarni SK. Modulatory effect of sildenafil in diabetes and electroconvulsive shock-induced cognitive dysfunction in rats. Pharmacol Rep. 2006;58(3):373–80. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

