Independent and cooperative roles of the Mek/ERK1/2-MAPK and PI3K/Akt/mTOR pathways during developmental myelination and in adulthood
- PMID: 30761608
- PMCID: PMC6571146
- DOI: 10.1002/glia.23602
Independent and cooperative roles of the Mek/ERK1/2-MAPK and PI3K/Akt/mTOR pathways during developmental myelination and in adulthood
Abstract
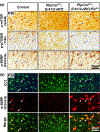
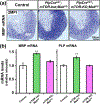
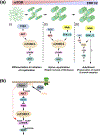
Multiple extracellular and intracellular signals regulate the functions of oligodendrocytes as they progress through the complex process of developmental myelination and then maintain a functionally intact myelin sheath throughout adult life, preserving the integrity of the axons. Recent studies suggest that Mek/ERK1/2-MAPK and PI3K/Akt/mTOR intracellular signaling pathways play important, often overlapping roles in the regulation of myelination. However, it remains poorly understood whether they function independently, sequentially, or converge using a common mechanism to facilitate oligodendrocyte differentiation, myelin growth, and maintenance. To address these questions, we analyzed multiple genetically modified mice and asked whether the deficits due to the conditional loss-of-function of ERK1/2 or mTOR could be abrogated by simultaneous constitutive activation of PI3K/Akt or Mek, respectively. From these studies, we concluded that while PI3K/Akt, not Mek/ERK1/2, plays a key role in promoting oligodendrocyte differentiation and timely initiation of myelination through mTORC1 signaling, Mek/ERK1/2-MAPK functions largely independently of mTORC1 to preserve the integrity of the myelinated axons during adulthood. However, to promote the efficient growth of the myelin sheath, these two pathways cooperate with each other converging at the level of mTORC1, both in the context of normal developmental myelination or following forced reactivation of the myelination program during adulthood. Thus, Mek/ERK1/2-MAPK and the PI3K/Akt/mTOR signaling pathways work both independently and cooperatively to maintain a finely tuned, temporally regulated balance as oligodendrocytes progress through different phases of developmental myelination into adulthood. Therapeutic strategies aimed at targeting remyelination in demyelinating diseases are expected to benefit from these findings.
Keywords: differentiation; myelin; myelination; myelinogenesis; oligodendrocyte.
© 2019 Wiley Periodicals, Inc.
Figures





References
-
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, & Cohen P (1997). Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Current Biology, 7(4), 261–269. - PubMed
-
- Bansal R, Magge S, & Winkler S (2003). Specific inhibitor of FGF receptor signaling: FGF-2-mediated effects on proliferation, differentiation, and MAPK activation are inhibited by PD173074 in oligodendrocyte lineage cells. Journal of Neuroscience Research, 74(4), 486–493. 10.1002/jnr.10773 - DOI - PubMed
-
- Baron W, Metz B, Bansal R, Hoekstra D, & de Vries H (2000). PDGF and FGF-2 signaling in oligodendrocyte progenitor cells: Regulation of proliferation and differentiation by multiple intracellular signaling pathways. Molecular and Cellular Neuroscience, 15(3), 314–329. 10.1006/mcne.1999.0827 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

