miR-181c-5p mediates simulated microgravity-induced impaired osteoblast proliferation by promoting cell cycle arrested in the G2 phase
- PMID: 30761733
- PMCID: PMC6484313
- DOI: 10.1111/jcmm.14220
miR-181c-5p mediates simulated microgravity-induced impaired osteoblast proliferation by promoting cell cycle arrested in the G2 phase
Abstract
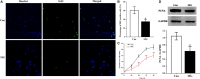
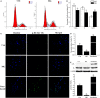
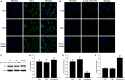
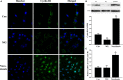
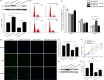
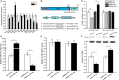
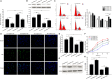
Impaired osteoblast proliferation plays fundamental roles in microgravity-induced bone loss, and cell cycle imbalance may result in abnormal osteoblast proliferation. However, whether microgravity exerts an influence on the cell cycle in osteoblasts or what mechanisms may underlie such an effect remains to be fully elucidated. Herein, we confirmed that simulated microgravity inhibits osteoblast proliferation. Then, we investigated the effect of mechanical unloading on the osteoblast cell cycle and found that simulated microgravity arrested the osteoblast cell cycle in the G2 phase. In addition, our data showed that cell cycle arrest in osteoblasts from simulated microgravity was mainly because of decreased cyclin B1 expression. Furthermore, miR-181c-5p directly inhibited cyclin B1 protein translation by binding to a target site in the 3'UTR. Lastly, we demonstrated that inhibition of miR-181c-5p partially counteracted cell cycle arrest and decreased the osteoblast proliferation induced by simulated microgravity. In conclusion, our study demonstrates that simulated microgravity inhibits cell proliferation and induces cell cycle arrest in the G2 phase in primary mouse osteoblasts partially through the miR-181c-5p/cyclin B1 pathway. This work may provide a novel mechanism of microgravity-induced detrimental effects on osteoblasts and offer a new avenue to further investigate bone loss induced by mechanical unloading.
Keywords: cell cycle; cell proliferation; cyclin B1; miR-181c-5p; osteoblast; simulated microgravity.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures
References
-
- Aisha MD, Nor‐Ashikin MN, Sharaniza AB, et al. Orbital fluid shear stress promotes osteoblast metabolism, proliferation and alkaline phosphates activity in vitro. Exp Cell Res. 2015;337:87‐93. - PubMed
-
- Saito M, Soshi S, Fujii K. Effect of hyper‐ and microgravity on collagen post‐translational controls of MC3T3‐E1 osteoblasts. J Bone Miner Res. 2003;18:1695‐1705. - PubMed
-
- Rittweger J, Frost HM, Schiessl H, et al. Muscle atrophy and bone loss after 90 days' bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone. 2005;36:1019‐1029. - PubMed
-
- Bucaro MA, Fertala J, Adams CS, et al. Bone cell survival in microgravity: evidence that modeled microgravity increases osteoblast sensitivity to apoptogens. Ann NY Acad Sci. 2004;1027:64‐73. - PubMed
-
- Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int. 1995;57:344‐358. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

