Reasons for low uptake of a psychological intervention offered to cancer survivors with elevated depressive symptoms
- PMID: 30762273
- PMCID: PMC6593801
- DOI: 10.1002/pon.5029
Reasons for low uptake of a psychological intervention offered to cancer survivors with elevated depressive symptoms
Erratum in
-
Erratum.Psychooncology. 2019 Sep;28(9):1942. doi: 10.1002/pon.5184. Epub 2019 Jul 30. Psychooncology. 2019. PMID: 31489758 Free PMC article. No abstract available.
Abstract
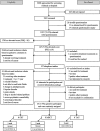
Objective: In line with screening guidelines, cancer survivors were consecutively screened on depressive symptoms (as part of standard care), with those reporting elevated levels of symptoms offered psychological care as part of a trial. Because of the low uptake, no conclusions could be drawn about the interventions' efficacy. Given the trial set-up (following screening guidelines and strict methodological quality criteria), we believe that this observational study reporting the flow of participation, reasons for and characteristics associated with nonparticipation, adds to the debate about the feasibility and efficiency of screening guidelines.
Methods: Two thousand six hundred eight medium- to long-term cancer survivors were consecutively screened on depressive symptoms using the Patient Health Questionnaire-9 (PHQ-9). Those with moderate depressive symptoms (PHQ-9 ≥ 10) were contacted and informed about the trial. Patient flow and reasons for nonparticipation were carefully monitored.
Results: One thousand thirty seven survivors (74.3%) returned the questionnaire, with 147 (7.6%) reporting moderate depressive symptoms. Of this group, 49 survivors (33.3%) were ineligible, including 26 survivors (17.7%) already receiving treatment and another 44 survivors (30.0%) reporting no need for treatment. Only 25 survivors (1.0%) participated in the trial.
Conclusion: Of the approached survivors for screening, only 1% was eligible and interested in receiving psychological care as part of our trial. Four reasons for nonparticipation were: nonresponse to screening, low levels of depressive symptoms, no need, or already receiving care. Our findings question whether to spend the limited resources in psycho-oncological care on following screening guidelines and the efficiency of using consecutive screening for trial recruitment in cancer survivors.
Keywords: CONSORT; cancer; cancer survivors; consecutive screening; depression; oncology; randomized controlled trial; recruitment; screening; screening guidelines.
© 2019 The Authors. Psycho-Oncology Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no potential conflicts of interest to report.
Figures
References
-
- Faller H, Schuler M, Richard M, Heckl U, Weis J, Ku R. Effects of psycho‐oncologic interventions on emotional distress and quality of life in adult patients with cancer: systematic review and meta‐analysis. J Clin Oncol. 2017;31(6):782‐793. - PubMed
-
- Jacobsen PB, Jim HS. Psychosocial interventions for anxiety and depression in adult cancer patients: achievements and challenges. CA ‐ A Cancer J Clin. 2008;58(4):214‐230. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical