Long-term efficacy and safety of biosimilar infliximab (CT-P13) after switching from originator infliximab: open-label extension of the NOR-SWITCH trial
- PMID: 30762274
- PMCID: PMC6850326
- DOI: 10.1111/joim.12880
Long-term efficacy and safety of biosimilar infliximab (CT-P13) after switching from originator infliximab: open-label extension of the NOR-SWITCH trial
Abstract
Background and objectives: The 52-week, randomized, double-blind, noninferiority, government-funded NOR-SWITCH trial demonstrated that switching from infliximab originator to less expensive biosimilar CT-P13 was not inferior to continued treatment with infliximab originator. The NOR-SWITCH extension trial aimed to assess efficacy, safety and immunogenicity in patients on CT-P13 throughout the 78-week study period (maintenance group) versus patients switched to CT-P13 at week 52 (switch group). The primary outcome was disease worsening during follow-up based on disease-specific composite measures.
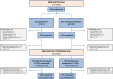
Methods: Patients were recruited from 24 Norwegian hospitals, 380 of 438 patients who completed the main study: 197 in the maintenance group and 183 in the switch group. In the full analysis set, 127 (33%) had Crohn's disease, 80 (21%) ulcerative colitis, 67 (18%) spondyloarthritis, 55 (15%) rheumatoid arthritis, 20 (5%) psoriatic arthritis and 31 (8%) chronic plaque psoriasis.
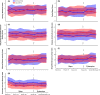
Results: Baseline characteristics were similar in the two groups at the time of switching (week 52). Disease worsening occurred in 32 (16.8%) patients in the maintenance group vs. 20 (11.6%) in the switch group (per-protocol set). Adjusted risk difference was 5.9% (95% CI -1.1 to 12.9). Frequency of adverse events, anti-drug antibodies, changes in generic disease variables and disease-specific composite measures were comparable between arms. The study was inadequately powered to detect noninferiority within individual diseases.
Conclusion: The NOR-SWITCH extension showed no difference in safety and efficacy between patients who maintained CT-P13 and patients who switched from originator infliximab to CT-P13, supporting that switching from originator infliximab to CT-P13 is safe and efficacious.
Trial registration: ClinicalTrials.gov NCT02148640.
Keywords: biosimilar; chronic inflammatory disease; drug costs; health economics; infliximab; switching.
© 2019 The Authors. Journal of Internal Medicine published by John Wiley & Sons Ltd on behalf of Association for Publication of The Journal of Internal Medicine.
Conflict of interest statement
GLG reports personal fees from AbbVie, Biogen, Eli Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, Orion Pharma, Celltrion and Boehringer Ingelheim. KKJ reports personal fees from Tillott, Intercept and Celltrion. NB reports personal fees received from Orion Pharma, Roche, Napp Pharmaceuticals, Pfizer and Takeda. ICO reports grants from Norwegian Ministry of Health and Care Services during the conduct of the study and personal fees from Pfizer. EAH reports grants from AbbVie, Pfizer, UCB, Roche and MSD. IPB reports personal fees from AbbVie, MSD, Takeda, Hospira and Ferring. KEAL reports grants from MSD and personal fees from Takeda, Orion, AbbVie, Pfizer and MSD. KST reports personal fees and nonfinancial support from AbbVie, Orion, Novartis, Janssen, Celgene, Mundipharma, Pfizer, MSD and Shire and nonfinancial support from CSL Behring. CM reports personal fees from Novartis Norge AS, LEO Pharma AS, ACO Hud Norge AS, Celgene AS, AbbVie and Galderma Nordic AB. JJ reports personal fees from MSD, AbbVie, Celltrion, Orion Pharma, Takeda, Napp Pharm, AstroPharma, Hikma and Pfizer. TKK reports grants from Norwegian Ministry of Health and Care Services during the conduct of the study and personal fees from AbbVie, Biogen, BMS, Boehringer Ingelheim, Celltrion, Eli Lilly, Epirus, Janssen, Merck‐Serono, MSD, Mundipharma, Novartis, Oktal, Orion Pharma, Hospira/Pfizer, Roche, Sandoz and UCB Pharma.
Figures

Comment in
-
A 'wind of change' to biosimilars: The NOR-SWITCH trial and its extension.J Intern Med. 2019 Jun;285(6):693-695. doi: 10.1111/joim.12896. Epub 2019 Apr 16. J Intern Med. 2019. PMID: 30990229 No abstract available.
References
-
- Putrik P, Ramiro S, Kvien TK, Sokka T, Uhlig T, Boonen A. Variations in criteria regulating treatment with reimbursed biologic DMARDs across European countries. Are differences related to country's wealth? Ann Rheum Dis 2014; 73: 2010–21. - PubMed
-
- Yoo DH, Hrycaj P, Miranda P et al A randomised, double‐blind, parallel‐group study to demonstrate equivalence in efficacy and safety of CT‐P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis 2013; 72: 1613–20. - PMC - PubMed
-
- Park W, Hrycaj P, Jeka S et al A randomised, double‐blind, multicentre, parallel‐group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT‐P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis 2013; 72: 1605–12. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

