SSRP1 promotes colorectal cancer progression and is negatively regulated by miR-28-5p
- PMID: 30762286
- PMCID: PMC6484412
- DOI: 10.1111/jcmm.14134
SSRP1 promotes colorectal cancer progression and is negatively regulated by miR-28-5p
Retraction in
-
Retraction: SSRP1 Promotes Colorectal Cancer Progression and is Negatively Regulated by miR-28-5p.J Cell Mol Med. 2024 Sep;28(17):e70048. doi: 10.1111/jcmm.70048. J Cell Mol Med. 2024. PMID: 39267240 Free PMC article.
Abstract
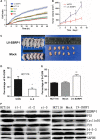
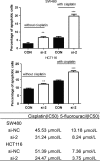
In this study, microarray data analysis, real-time quantitative PCR and immunohistochemistry were used to detect the expression levels of SSRP1 in colorectal cancer (CRC) tissue and in corresponding normal tissue. The association between structure-specific recognition protein 1 (SSRP1) expression and patient prognosis was examined by Kaplan-Meier analysis. SSRP1 was knocked down and overexpressed in CRC cell lines, and its effects on proliferation, cell cycling, migration, invasion, cellular energy metabolism, apoptosis, chemotherapeutic drug sensitivity and cell phenotype-related molecules were assessed. The growth of xenograft tumours in nude mice was also assessed. MiRNAs that potentially targeted SSRP1 were determined by bioinformatic analysis, Western blotting and luciferase reporter assays. We showed that SSRP1 mRNA levels were significantly increased in CRC tissue. We also confirmed that this upregulation was related to the terminal tumour stage in CRC patients, and high expression levels of SSRP1 predicted shorter disease-free survival and faster relapse. We also found that SSRP1 modulated proliferation, metastasis, cellular energy metabolism and the epithelial-mesenchymal transition in CRC. Furthermore, SSRP1 induced apoptosis and SSRP1 knockdown augmented the sensitivity of CRC cells to 5-fluorouracil and cisplatin. Moreover, we explored the molecular mechanisms accounting for the dysregulation of SSRP1 in CRC and identified microRNA-28-5p (miR-28-5p) as a direct upstream regulator of SSRP1. We concluded that SSRP1 promotes CRC progression and is negatively regulated by miR-28-5p.
Keywords: SSRP1; colorectal cancer; microRNA; progression.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors confirm that there are no conflicts of interests.
Figures



References
-
- Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101‐2114. - PubMed
-
- Huang D, Sun W, Zhou Y, et al. Mutations of key driver genes in colorectal cancer progression and metastasis. Cancer Metastasis Rev. 2018;37:173‐187. - PubMed
-
- Mathot L, Kundu S, Ljungström V, et al. Somatic ephrin receptor mutations are associated with metastasis in primary colorectal cancer. Cancer Res. 2017;77:1730‐1740. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

