The role of somatic mutational events in the pathogenesis of epilepsy
- PMID: 30762606
- PMCID: PMC6424360
- DOI: 10.1097/WCO.0000000000000667
The role of somatic mutational events in the pathogenesis of epilepsy
Abstract
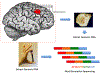
Purpose of review: There has been rapid progress in defining novel causative gene variants responsible for a large spectrum of human epilepsy syndromes and subtypes. Of particular interest is the discovery that somatic mutations, for example, noninherited mutations occurring in neuroglial progenitor cells during embryonic brain development, are highly linked to malformations of cortical development (MCD) such as focal cortical dysplasia (FCD) type II and hemimegalencephaly.
Recent findings: Somatic gene variants have been identified in genes encoding regulatory proteins within the mechanistic target of rapamycin (mTOR) signaling cascade and have thus comprised the group classified as mTORopathies. FCD II and hemimegalencephaly often result from mutations in identical genes suggesting that these are spectrum disorders. An exciting recent development has been the identification of somatic mutations causing both FCD Ia and nonlesional neocortical epilepsy.
Summary: Defining somatic gene mutations in brain tissue specimens has shed new light on how MCD form and the mechanisms of epileptogenesis associated with MCD. Trials of mTOR inhibitors in tuberous sclerosis complex have demonstrated that inhibition of mTOR activation in mTORopathies can reduce seizure frequency. New somatic mutations found for a variety of epilepsy syndromes may provide new targets for clinical therapeutics.
Conflict of interest statement
There are no conflicts of interest.
Figures


References
-
- Nolan D, Fink J. Genetics of epilepsy. Handb Clin Neurol 2018; 148: 467–491 - PubMed
-
-
Iffland PH 2nd, Crino PB. Focal Cortical Dysplasia: Gene mutations, cell signaling, and therapeutic implications Annu Rev Pathol. 2017; 12:547–571
• A comprehensive review of the pathogenesis of focal cortical dysplasia and related malformations.
-
-
-
Najm IM, Sarnat HB, Blümcke I. Review: The international consensus classification of Focal Cortical Dysplasia - a critical update 2018. Neuropathol Appl Neurobiol 2018; 44: 18–31
• An outstanding review of how cortical dysplasias are classified.
-
-
- Peron A, Au KS, Northrup H. Genetics, genomics, and genotype-phenotype correlations of TSC: Insights for clinical practice. Am J Med Genet C Semin Med Genetic 2018; 178: 281–290 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

