Alterations in fecal short-chain fatty acids in patients with irritable bowel syndrome: A systematic review and meta-analysis
- PMID: 30762787
- PMCID: PMC6408019
- DOI: 10.1097/MD.0000000000014513
Alterations in fecal short-chain fatty acids in patients with irritable bowel syndrome: A systematic review and meta-analysis
Abstract
Background: Recent studies indicate that gut microbiota disorders potentially contribute to the pathogenesis of irritable bowel syndrome (IBS), which can be partly reflected by fecal short-chain fatty acids (SCFAs) generated from gut microbiota. Previous studies on SCFA alterations in patients with IBS have yielded conflicting results. No prior systematic review has been conducted on the alterations in fecal SCFAs in IBS patients.
Aims: We performed a meta-analysis to explore and clarify alterations in fecal SCFAs in IBS patients.
Methods: Case-control studies, randomized controlled trials (RCTs), and self-controlled studies were identified through electronic database searches. The standardized mean difference (SMD) with 95% confidence interval (CI) in fecal SCFA levels between different groups was calculated.
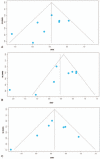
Results: The proportion of fecal propionate in patients with IBS was significantly higher than in healthy controls (HCs) (SMD = 0.44, 95% CI = 0.12, 0.76). A subgroup analysis showed that the concentration of fecal propionate (SMD = -0.91, 95% CI = -1.41, -0.41) and butyrate (SMD = -0.53, 95% CI = -1.01, -0.04) in patients with constipation-predominant IBS (IBS-C) was significantly lower than that in HCs, and the concentration of fecal butyrate in patients with diarrhea-predominant IBS (IBS-D) was higher than that in HCs (SMD = 0.34, 95% CI = 0.00, 0.67). Finally, we found that restricted diets correlated with fecal butyrate reduction in IBS (SMD = -0.26, 95% CI = -0.51, -0.01).
Conclusions: In terms of fecal SCFAs, there were differences between patients with IBS and HCs. In IBS-C patients, propionate and butyrate were reduced, whereas butyrate was increased in IBS-D patients in comparison to HCs. Propionate and butyrate could be used as biomarkers for IBS diagnosis.
Conflict of interest statement
No potential conflicts of interest relevant to this article were reported.
Figures



Similar articles
-
Changes in Fecal Short-Chain Fatty Acids in IBS Patients and Effects of Different Interventions: A Systematic Review and Meta-Analysis.Nutrients. 2024 May 31;16(11):1727. doi: 10.3390/nu16111727. Nutrients. 2024. PMID: 38892659 Free PMC article.
-
Fecal bacteria and short-chain fatty acids in irritable bowel syndrome: Relations to subtype.Neurogastroenterol Motil. 2024 Sep;36(9):e14854. doi: 10.1111/nmo.14854. Epub 2024 Jun 30. Neurogastroenterol Motil. 2024. PMID: 38946176
-
Associations of Habitual Dietary Intake With Fecal Short-Chain Fatty Acids and Bowel Functions in Irritable Bowel Syndrome.J Clin Gastroenterol. 2022 Mar 1;56(3):234-242. doi: 10.1097/MCG.0000000000001521. J Clin Gastroenterol. 2022. PMID: 33780215 Free PMC article.
-
Fecal short-chain fatty acids in patients with diarrhea-predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation.J Pediatr Gastroenterol Nutr. 1996 Oct;23(3):280-6. doi: 10.1097/00005176-199610000-00013. J Pediatr Gastroenterol Nutr. 1996. PMID: 8890079
-
Alterations in short-chain fatty acids and serotonin in irritable bowel syndrome: a systematic review and meta-analysis.BMC Gastroenterol. 2021 Jan 6;21(1):14. doi: 10.1186/s12876-020-01577-5. BMC Gastroenterol. 2021. PMID: 33407171 Free PMC article.
Cited by
-
Altered epithelial barrier functions in the colon of patients with spina bifida.Sci Rep. 2022 May 3;12(1):7196. doi: 10.1038/s41598-022-11289-3. Sci Rep. 2022. PMID: 35505001 Free PMC article. Clinical Trial.
-
Short-chain fatty acids and insulin sensitivity: a systematic review and meta-analysis.Nutr Rev. 2024 Jan 10;82(2):193-209. doi: 10.1093/nutrit/nuad042. Nutr Rev. 2024. PMID: 37290429 Free PMC article.
-
Changes in Fecal Short-Chain Fatty Acids in IBS Patients and Effects of Different Interventions: A Systematic Review and Meta-Analysis.Nutrients. 2024 May 31;16(11):1727. doi: 10.3390/nu16111727. Nutrients. 2024. PMID: 38892659 Free PMC article.
-
Effects of a Protease Inhibitor Camostat Mesilate on Gut Microbial Function in Patients with Irritable Bowel Syndrome: A Pilot Randomized Placebo-Controlled Study.Digestion. 2025;106(4):265-276. doi: 10.1159/000542758. Epub 2024 Nov 25. Digestion. 2025. PMID: 39586243 Free PMC article. Clinical Trial.
-
The Association between Dysbiosis and Neurological Conditions Often Manifesting with Chronic Pain.Biomedicines. 2023 Mar 1;11(3):748. doi: 10.3390/biomedicines11030748. Biomedicines. 2023. PMID: 36979726 Free PMC article. Review.
References
-
- Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 2006;130:1377–90. - PubMed
-
- Müller-Lissner S, Kamm MA, Musoglu A, et al. Safety, tolerability, and efficacy of tegaserod over 13 months in patients with chronic constipation. Am J Gastroenterol 2006;101:2558–69. quiz 2671. - PubMed
-
- Müller-Lissner S, Holtmann G, Rueegg P, et al. Tegaserod is effective in the initial and retreatment of irritable bowel syndrome with constipation. Aliment Pharmacol Ther 2005;21:11–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

