Decolonization to Reduce Postdischarge Infection Risk among MRSA Carriers
- PMID: 30763195
- PMCID: PMC6475519
- DOI: 10.1056/NEJMoa1716771
Decolonization to Reduce Postdischarge Infection Risk among MRSA Carriers
Abstract
Background: Hospitalized patients who are colonized with methicillin-resistant Staphylococcus aureus (MRSA) are at high risk for infection after discharge.
Methods: We conducted a multicenter, randomized, controlled trial of postdischarge hygiene education, as compared with education plus decolonization, in patients colonized with MRSA (carriers). Decolonization involved chlorhexidine mouthwash, baths or showers with chlorhexidine, and nasal mupirocin for 5 days twice per month for 6 months. Participants were followed for 1 year. The primary outcome was MRSA infection as defined according to Centers for Disease Control and Prevention (CDC) criteria. Secondary outcomes included MRSA infection determined on the basis of clinical judgment, infection from any cause, and infection-related hospitalization. All analyses were performed with the use of proportional-hazards models in the per-protocol population (all participants who underwent randomization, met the inclusion criteria, and survived beyond the recruitment hospitalization) and as-treated population (participants stratified according to adherence).
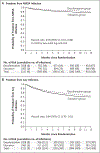
Results: In the per-protocol population, MRSA infection occurred in 98 of 1063 participants (9.2%) in the education group and in 67 of 1058 (6.3%) in the decolonization group; 84.8% of the MRSA infections led to hospitalization. Infection from any cause occurred in 23.7% of the participants in the education group and 19.6% of those in the decolonization group; 85.8% of the infections led to hospitalization. The hazard of MRSA infection was significantly lower in the decolonization group than in the education group (hazard ratio, 0.70; 95% confidence interval [CI], 0.52 to 0.96; P=0.03; number needed to treat to prevent one infection, 30; 95% CI, 18 to 230); this lower hazard led to a lower risk of hospitalization due to MRSA infection (hazard ratio, 0.71; 95% CI, 0.51 to 0.99). The decolonization group had lower likelihoods of clinically judged infection from any cause (hazard ratio, 0.83; 95% CI, 0.70 to 0.99) and infection-related hospitalization (hazard ratio, 0.76; 95% CI, 0.62 to 0.93); treatment effects for secondary outcomes should be interpreted with caution owing to a lack of prespecified adjustment for multiple comparisons. In as-treated analyses, participants in the decolonization group who adhered fully to the regimen had 44% fewer MRSA infections than the education group (hazard ratio, 0.56; 95% CI, 0.36 to 0.86) and had 40% fewer infections from any cause (hazard ratio, 0.60; 95% CI, 0.46 to 0.78). Side effects (all mild) occurred in 4.2% of the participants.
Conclusions: Postdischarge MRSA decolonization with chlorhexidine and mupirocin led to a 30% lower risk of MRSA infection than education alone. (Funded by the AHRQ Healthcare-Associated Infections Program and others; ClinicalTrials.gov number, NCT01209234 .).
Figures

Comment in
-
Postdischarge Infection Risk among MRSA Carriers.N Engl J Med. 2019 May 30;380(22):2182. doi: 10.1056/NEJMc1903763. N Engl J Med. 2019. PMID: 31141647 No abstract available.
References
-
- Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34:1–14. - PubMed
-
- von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med 2001;344:11–6. - PubMed
-
- Methicillin-resistant Staphylococcus aureus: information for patients Atlanta: Centers for Disease Control and Prevention, 2016. (https://www.cdc.gov/mrsa/healthcare/patient/index.html).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
