PD-L1 on invasive fibroblasts drives fibrosis in a humanized model of idiopathic pulmonary fibrosis
- PMID: 30763282
- PMCID: PMC6482997
- DOI: 10.1172/jci.insight.125326
PD-L1 on invasive fibroblasts drives fibrosis in a humanized model of idiopathic pulmonary fibrosis
Abstract
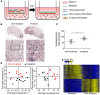
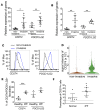
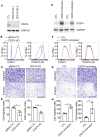
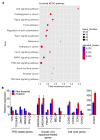
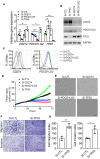
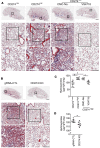
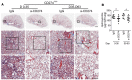
Idiopathic pulmonary fibrosis (IPF) is a progressive disease with unremitting extracellular matrix deposition, leading to a distortion of pulmonary architecture and impaired gas exchange. Fibroblasts from IPF patients acquire an invasive phenotype that is essential for progressive fibrosis. Here, we performed RNA sequencing analysis on invasive and noninvasive fibroblasts and found that the immune checkpoint ligand CD274 (also known as PD-L1) was upregulated on invasive lung fibroblasts and was required for the invasive phenotype of lung fibroblasts, is regulated by p53 and FAK, and drives lung fibrosis in a humanized IPF model in mice. Activating CD274 in IPF fibroblasts promoted invasion in vitro and pulmonary fibrosis in vivo. CD274 knockout in IPF fibroblasts and targeting CD274 by FAK inhibition or CD274-neutralizing antibodies blunted invasion and attenuated fibrosis, suggesting that CD274 may be a novel therapeutic target in IPF.
Keywords: Cell migration/adhesion; Fibrosis; Pulmonology; Respiration; Therapeutics.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

