Update: Influenza Activity - United States, September 30, 2018-February 2, 2019
- PMID: 30763296
- PMCID: PMC6375659
- DOI: 10.15585/mmwr.mm6806a1
Update: Influenza Activity - United States, September 30, 2018-February 2, 2019
Abstract
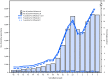
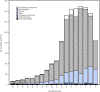
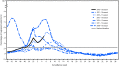
CDC collects, compiles, and analyzes data on influenza activity and viruses in the United States. During September 30, 2018-February 2, 2019,* influenza activity† in the United States was low during October and November, increased in late December, and remained elevated through early February. As of February 2, 2019, this has been a low-severity influenza season (1), with a lower percentage of outpatient visits for influenza-like illness (ILI), lower rates of hospitalization, and fewer deaths attributed to pneumonia and influenza, compared with recent seasons. Influenza-associated hospitalization rates among children are similar to those observed in influenza A(H1N1)pdm09 predominant seasons; 28 influenza-associated pediatric deaths occurring during the 2018-19 season have been reported to CDC. Whereas influenza A(H1N1)pdm09 viruses predominated in most areas of the country, influenza A(H3N2) viruses have predominated in the southeastern United States, and in recent weeks accounted for a growing proportion of influenza viruses detected in several other regions. Small numbers of influenza B viruses (<3% of all influenza-positive tests performed by public health laboratories) also were reported. The majority of the influenza viruses characterized antigenically are similar to the cell culture-propagated reference viruses representing the 2018-19 Northern Hemisphere influenza vaccine viruses. Health care providers should continue to offer and encourage vaccination to all unvaccinated persons aged ≥6 months as long as influenza viruses are circulating. Finally, regardless of vaccination status, it is important that persons with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at high risk for influenza complications be treated with antiviral medications.
Conflict of interest statement
All authors have completed and submitted the ICMJE form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
References
-
- World Health Organization. Laboratory methodologies for testing the antiviral susceptibility of influenza viruses: neuraminidase inhibitor (NAI). Geneva, Switzerland: World Health Organization; 2019. https://www.who.int/influenza/gisrs_laboratory/antiviral_susceptibility/...
-
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018;67(No. RR-3). 10.15585/mmwr.rr6703a1 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

