The Wilms' tumor suppressor gene regulates pancreas homeostasis and repair
- PMID: 30763305
- PMCID: PMC6392337
- DOI: 10.1371/journal.pgen.1007971
The Wilms' tumor suppressor gene regulates pancreas homeostasis and repair
Abstract
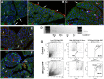
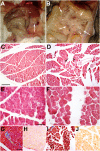
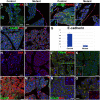
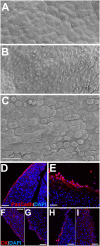
The Wilms' tumor suppressor gene (Wt1) encodes a zinc finger transcription factor that plays an essential role in the development of kidneys, gonads, spleen, adrenals and heart. Recent findings suggest that WT1 could also be playing physiological roles in adults. Systemic deletion of WT1 in mice provokes a severe deterioration of the exocrine pancreas, with mesothelial disruption, E-cadherin downregulation, disorganization of acinar architecture and accumulation of ascitic transudate. Despite this extensive damage, pancreatic stellate cells do not become activated and lose their canonical markers. We observed that pharmacological induction of pancreatitis in normal mice provokes de novo expression of WT1 in pancreatic stellate cells, concomitant with their activation. When pancreatitis was induced in mice after WT1 ablation, pancreatic stellate cells expressed WT1 and became activated, leading to a partial rescue of the acinar structure and the quiescent pancreatic stellate cell population after recovery from pancreatitis. We propose that WT1 modulates through the RALDH2/retinoic acid axis the restabilization of a part of the pancreatic stellate cell population and, indirectly, the repair of the pancreatic architecture, since quiescent pancreatic stellate cells are required for pancreas stability and repair. Thus, we suggest that WT1 plays novel and essential roles for the homeostasis of the adult pancreas and, through its upregulation in pancreatic stellate cells after a damage, for pancreatic regeneration. Due to the growing importance of the pancreatic stellate cells in physiological and pathophysiological conditions, these novel roles can be of translational relevance.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures