A comparison of the Muenster, SIOP Boston, Brock, Chang and CTCAEv4.03 ototoxicity grading scales applied to 3,799 audiograms of childhood cancer patients treated with platinum-based chemotherapy
- PMID: 30763334
- PMCID: PMC6375552
- DOI: 10.1371/journal.pone.0210646
A comparison of the Muenster, SIOP Boston, Brock, Chang and CTCAEv4.03 ototoxicity grading scales applied to 3,799 audiograms of childhood cancer patients treated with platinum-based chemotherapy
Abstract
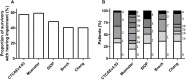
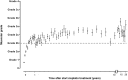
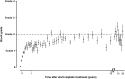
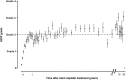
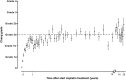
Childhood cancer patients treated with platinums often develop hearing loss and the degree is classified according to different scales globally. Our objective was to compare concordance between five well-known ototoxicity scales used for childhood cancer patients. Audiometric test results (n = 654) were evaluated longitudinally and graded according Brock, Chang, International Society of Pediatric Oncology (SIOP) Boston, Muenster scales and the U.S. National Cancer Institute Common Technology Criteria for Adverse Events (CTCAE) version 4.03. Adverse effects of grade 2, 3 and 4 are considered to reflect a degree of hearing loss sufficient to interfere with day-to-day communication (> = Chang grade 2a; > = Muenster grade 2b). We term this "deleterious hearing loss". A total number of 3,799 audiograms were evaluated. The prevalence of deleterious hearing loss according to the last available audiogram of each patient was 59.3% (388/654) according to Muenster, 48.2% (315/653) according to SIOP, 40.5% (265/652) according to Brock, 40.3% (263/652) according to Chang, and 57.5% (300/522) according to CTCAEv4.03. Overall concordance between the scales ranged from ĸ = 0.636 (Muenster vs. Chang) to ĸ = 0.975 (Brock vs. Chang). Muenster detected hearing loss the earliest in time, followed by Chang, SIOP and Brock. Generally good concordance between the scales was observed but there is still diversity in definitions of functional outcomes, such as differences in distribution levels of severity of hearing loss, and additional intermediate scales taking into account losses <40 dB as well. Regardless of the scale used, hearing function decreases over time and therefore, close monitoring of hearing function at baseline and with each cycle of platinum therapy should be conducted.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Bess FH, Dodd-Murphy J, Parker RA. Children with minimal sensorineural hearing loss: prevalence, educational performance, and functional status. Ear Hear. 1998;19(5):339–54. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

