Spatiotemporal expression of the putative MdtABC efflux pump of Phtotorhabdus luminescens occurs in a protease-dependent manner during insect infection
- PMID: 30763358
- PMCID: PMC6375597
- DOI: 10.1371/journal.pone.0212077
Spatiotemporal expression of the putative MdtABC efflux pump of Phtotorhabdus luminescens occurs in a protease-dependent manner during insect infection
Abstract
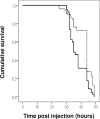
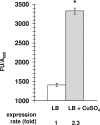
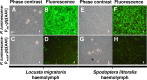
Photorhabdus luminescens is an enterobacterium establishing a mutualistic symbiosis with nematodes, that also kills insects after septicaemia and connective tissue colonization. The role of the bacterial mdtABC genes encoding a putative multidrug efflux system from the resistance/nodulation/cell division family was investigated. We showed that a mdtA mutant and the wild type had similar levels of resistance to antibiotics, antimicrobial peptides, metals, detergents and bile salts. The mdtA mutant was also as pathogenic as the wild-type following intrahaemocoel injection in Locusta migratoria, but had a slightly attenuated phenotype in Spodoptera littoralis. A transcriptional fusion of the mdtA promoter (PmdtA) and the green fluorescent protein (gfp) encoding gene was induced by copper in bacteria cultured in vitro. The PmdtA-gfp fusion was strongly induced within bacterial aggregates in the haematopoietic organ during late stages of infection in L. migratoria, whereas it was only weakly expressed in insect plasma throughout infection. A medium supplemented with haematopoietic organ extracts induced the PmdtA-gfp fusion ex vivo, suggesting that site-specific mdtABC expression resulted from insect signals from the haematopoietic organ. Finally, we showed that protease inhibitors abolished ex vivo activity of the PmdtA-gfp fusion in the presence of haematopoietic organ extracts, suggesting that proteolysis by-products play a key role in upregulating the putative MdtABC efflux pump during insect infection with P. luminescens.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Site-specific antiphagocytic function of the Photorhabdus luminescens type III secretion system during insect colonization.Cell Microbiol. 2005 Mar;7(3):363-71. doi: 10.1111/j.1462-5822.2004.00466.x. Cell Microbiol. 2005. PMID: 15679839
-
AcrAB, the major RND-type efflux pump of Photorhabdus laumondii, confers intrinsic multidrug-resistance and contributes to virulence in insects.Environ Microbiol Rep. 2021 Oct;13(5):637-648. doi: 10.1111/1758-2229.12974. Epub 2021 May 17. Environ Microbiol Rep. 2021. PMID: 34002534
-
Photorhabdus luminescens genes induced upon insect infection.BMC Genomics. 2008 May 19;9:229. doi: 10.1186/1471-2164-9-229. BMC Genomics. 2008. PMID: 18489737 Free PMC article.
-
Regulation of Phenotypic Switching and Heterogeneity in Photorhabdus luminescens Cell Populations.J Mol Biol. 2019 Nov 22;431(23):4559-4568. doi: 10.1016/j.jmb.2019.04.015. Epub 2019 Apr 22. J Mol Biol. 2019. PMID: 31022406 Review.
-
Novel antibiotic compounds produced by the insect pathogenic bacterium photorhabdus.Recent Pat Antiinfect Drug Discov. 2009 Jun;4(2):81-9. doi: 10.2174/157489109788490280. Recent Pat Antiinfect Drug Discov. 2009. PMID: 19519542 Review.
Cited by
-
Molecular Mechanisms of Bacterial Resistance to Antimicrobial Peptides in the Modern Era: An Updated Review.Microorganisms. 2024 Jun 21;12(7):1259. doi: 10.3390/microorganisms12071259. Microorganisms. 2024. PMID: 39065030 Free PMC article. Review.
-
Volatile Organic Compounds Produced by Kosakonia cowanii Cp1 Isolated from the Seeds of Capsicum pubescens R & P Possess Antifungal Activity.Microorganisms. 2023 Oct 4;11(10):2491. doi: 10.3390/microorganisms11102491. Microorganisms. 2023. PMID: 37894149 Free PMC article.
-
Metagenomics of antimicrobial and heavy metal resistance in the cecal microbiome of fattening pigs raised without antibiotics.Appl Environ Microbiol. 2021 Apr 15;87(8):e02684-20. doi: 10.1128/AEM.02684-20. Epub 2021 Feb 5. Appl Environ Microbiol. 2021. PMID: 33547058 Free PMC article.
-
Phylogenetic analysis reveals an ancient gene duplication as the origin of the MdtABC efflux pump.PLoS One. 2020 Feb 12;15(2):e0228877. doi: 10.1371/journal.pone.0228877. eCollection 2020. PLoS One. 2020. PMID: 32050009 Free PMC article.
-
Isolation, Identification, Antimicrobial Resistance, Genotyping, and Whole-Genome Sequencing Analysis of Salmonella Enteritidis Isolated from a Food-Poisoning Incident.Pol J Microbiol. 2024 Mar 4;73(1):69-89. doi: 10.33073/pjm-2024-008. eCollection 2024 Mar 1. Pol J Microbiol. 2024. PMID: 38437471 Free PMC article.
References
-
- Boemare NE. Biology, taxonomy and systematics of Photorhabdus and Xenorhabdus In: Gaugler R, editor. Entomopathogenic nematology. Wallingford, United Kingdom: CABI Publishing; 2002. p. 35–56.
-
- Eleftherianos I, Marokhazi J, Millichap PJ, Hodgkinson AJ, Sriboonlert A, ffrench-Constant RH, et al. Prior infection of Manduca sexta with non-pathogenic Escherichia coli elicits immunity to pathogenic Photorhabdus luminescens: roles of immune-related proteins shown by RNA interference. Insect Biochem Mol Biol. 2006;36(6):517–25. 10.1016/j.ibmb.2006.04.001 . - DOI - PubMed
-
- Brugirard-Ricaud K, Duchaud E, Givaudan A, Girard PA, Kunst F, Boemare N, et al. Site-specific antiphagocytic function of the Photorhabdus luminescens type III secretion system during insect colonization. Cell Microbiol. 2005;7(3):363–71. Epub 2005/02/01. CMI466 [pii] 10.1111/j.1462-5822.2004.00466.x . - DOI - PubMed
-
- Silva CP, Waterfield NR, Daborn PJ, Dean P, Chilver T, Au CP, et al. Bacterial infection of a model insect: Photorhabdus luminescens and Manduca sexta. Cell Microbiol. 2002;4(6):329–39. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

