Renal and vascular effects of kallikrein inhibition in a model of Lonomia obliqua venom-induced acute kidney injury
- PMID: 30763408
- PMCID: PMC6392336
- DOI: 10.1371/journal.pntd.0007197
Renal and vascular effects of kallikrein inhibition in a model of Lonomia obliqua venom-induced acute kidney injury
Abstract
Background: Lonomia obliqua venom is nephrotoxic and acute kidney injury (AKI) is the main cause of death among envenomed victims. Mechanism underlying L. obliqua-induced AKI involves renal hypoperfusion, inflammation, tubular necrosis and loss of glomerular filtration and tubular reabsorption capacities. In the present study, we aimed to investigate the contribution of kallikrein to the hemodynamic instability, inflammation and consequent renal and vascular impairment.
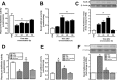
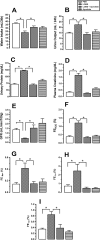
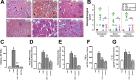
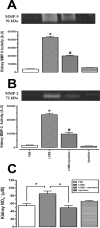
Methodology/principal findings: Addition of L. obliqua venom to purified prekallikrein and human plasma in vitro or to vascular smooth muscle cells (VSMC) in culture, was able to generate kallikrein in a dose-dependent manner. Injected in rats, the venom induced AKI and increased kallikrein levels in plasma and kidney. Kallikrein inhibition by aprotinin prevented glomerular injury and the decrease in glomerular filtration rate, restoring fluid and electrolyte homeostasis. The mechanism underlying these effects was associated to lowering renal inflammation, with decrease in pro-inflammatory cytokines and matrix metalloproteinase expression, reduced tubular degeneration, and protection against oxidative stress. Supporting the key role of kallikrein, we demonstrated that aprotinin inhibited effects directly associated with vascular injury, such as the generation of intracellular reactive oxygen species (ROS) and migration of VSMC induced by L. obliqua venom or by diluted plasma obtained from envenomed rats. In addition, kallikrein inhibition also ameliorated venom-induced blood incoagulability and decreased kidney tissue factor expression.
Conclusions/significance: These data indicated that kallikrein and consequently kinin release have a key role in kidney injury and vascular remodeling. Thus, blocking kallikrein may be a therapeutic alternative to control the progression of venom-induced AKI and vascular disturbances.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Veiga ABG, Berger M, Guimarães JA. Lonomia obliqua venom: pharmaco-toxicological effects and biotechnological perspectives In: de Lima ME, Pimenta AMC, Martin-Euclairte MF, Zingali RB, Rochat H, editors. Animal Toxins: State of the Art. Perspectives in Health and Biotechnology. Editora UFMG, Belo Horizonte; 2009. pp. 371–390.
-
- Berger M, Vieira MAR, Guimaraes JA. Acute Kidney Injury Induced by Snake and Arthropod Venoms In: Polenakovic M, editor. Renal Failure—The Facts. ISBN: 978953-51-0630-2, InTech-Open, Rijeka, Croatia; 2012. pp. 157–186.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

