Regulation of Nitric Oxide Production in the Developmental Programming of Hypertension and Kidney Disease
- PMID: 30764498
- PMCID: PMC6386843
- DOI: 10.3390/ijms20030681
Regulation of Nitric Oxide Production in the Developmental Programming of Hypertension and Kidney Disease
Abstract
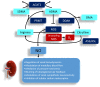
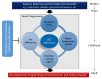
Development of the kidney can be altered in response to adverse environments leading to renal programming and increased vulnerability to the development of hypertension and kidney disease in adulthood. By contrast, reprogramming is a strategy shifting therapeutic intervention from adulthood to early life to reverse the programming processes. Nitric oxide (NO) is a key mediator of renal physiology and blood pressure regulation. NO deficiency is a common mechanism underlying renal programming, while early-life NO-targeting interventions may serve as reprogramming strategies to prevent the development of hypertension and kidney disease. This review will first summarize the regulation of NO in the kidney. We also address human and animal data supporting the link between NO system and developmental programming of hypertension and kidney disease. This will be followed by the links between NO deficiency and the common mechanisms of renal programming, including the oxidative stress, renin⁻angiotensin system, nutrient-sensing signals, and sex differences. Recent data from animal studies have suggested that interventions targeting the NO pathway could be reprogramming strategies to prevent the development of hypertension and kidney disease. Further clinical studies are required to bridge the gap between animal models and clinical trials in order to develop ideal NO-targeting reprogramming strategies and to be able to have a lifelong impact, with profound savings in the global burden of hypertension and kidney disease.
Keywords: asymmetric dimethylarginine; developmental origins of health and disease (DOHaD); hypertension; kidney disease; nitric oxide; nutrient-sensing signal; oxidative stress; renal programming; renin-angiotensin system; sex differences.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Baylis C., Beinder E., Suto T., August P. Recent insights into the roles of nitric oxide and renin-angiotensin in the pathophysiology of preeclamptic pregnancy. Semin. Nephrol. 1998;18:208–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

