Cord-Blood-Stem-Cell-Derived Conventional Dendritic Cells Specifically Originate from CD115-Expressing Precursors
- PMID: 30764500
- PMCID: PMC6406310
- DOI: 10.3390/cancers11020181
Cord-Blood-Stem-Cell-Derived Conventional Dendritic Cells Specifically Originate from CD115-Expressing Precursors
Abstract
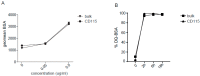
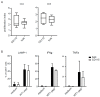
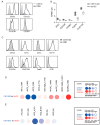
Dendritic cells (DCs) are professional antigen-presenting cells which instruct both the innate and adaptive immune systems. Once mature, they have the capacity to activate and prime naïve T cells for recognition and eradication of pathogens and tumor cells. These characteristics make them excellent candidates for vaccination strategies. Most DC vaccines have been generated from ex vivo culture of monocytes (mo). The use of mo-DCs as vaccines to induce adaptive immunity against cancer has resulted in clinical responses but, overall, treatment success is limited. The application of primary DCs or DCs generated from CD34⁺ stem cells have been suggested to improve clinical efficacy. Cord blood (CB) is a particularly rich source of CD34⁺ stem cells for the generation of DCs, but the dynamics and plasticity of the specific DC lineage development are poorly understood. Using flow sorting of DC progenitors from CB cultures and subsequent RNA sequencing, we found that CB-derived DCs (CB-DCs) exclusively originate from CD115⁺-expressing progenitors. Gene set enrichment analysis displayed an enriched conventional DC profile within the CD115-derived DCs compared with CB mo-DCs. Functional assays demonstrated that these DCs matured and migrated upon good manufacturing practice (GMP)-grade stimulation and possessed a high capacity to activate tumor-antigen-specific T cells. In this study, we developed a culture protocol to generate conventional DCs from CB-derived stem cells in sufficient numbers for vaccination strategies. The discovery of a committed DC precursor in CB-derived stem cell cultures further enables utilization of conventional DC-based vaccines to provide powerful antitumor activity and long-term memory immunity.
Keywords: DC precursor; DC subsets; cancer; cord blood; dendritic cell; vaccine.
Conflict of interest statement
The authors declare no conflicts of interest and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Feuerstein B., Berger T.G., Maczek C., Roder C., Schreiner D., Hirsch U., Haendle I., Leisgang W., Glaser A., Kuss O., et al. A method for the production of cryopreserved aliquots of antigen-preloaded, mature dendritic cells ready for clinical use. J. Immunol. Methods. 2000;245:15–29. doi: 10.1016/S0022-1759(00)00269-6. - DOI - PubMed
-
- Thurner B., Roder C., Dieckmann D., Heuer M., Kruse M., Glaser A., Keikavoussi P., Kampgen E., Bender A., Schuler G. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J. Immunol. Methods. 1999;223:1–15. doi: 10.1016/S0022-1759(98)00208-7. - DOI - PubMed
-
- Schadendorf D., Ugurel S., Schuler-Thurner B., Nestle F.O., Enk A., Brocker E.B., Grabbe S., Rittgen W., Edler L., Sucker A., et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: A randomized phase III trial of the DC study group of the DeCOG. Ann. Oncol. 2006;17:563–570. doi: 10.1093/annonc/mdj138. - DOI - PubMed
-
- Prue R.L., Vari F., Radford K.J., Tong H., Hardy M.Y., D’Rozario R., Waterhouse N.J., Rossetti T., Coleman R., Tracey C., et al. A phase I clinical trial of CD1c (BDCA-1)+ dendritic cells pulsed with HLA-A*0201 peptides for immunotherapy of metastatic hormone refractory prostate cancer. J. Immunother. 2015;38:71–76. doi: 10.1097/CJI.0000000000000063. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

