Ultrastructure in Transthyretin Amyloidosis: From Pathophysiology to Therapeutic Insights
- PMID: 30764529
- PMCID: PMC6466231
- DOI: 10.3390/biomedicines7010011
Ultrastructure in Transthyretin Amyloidosis: From Pathophysiology to Therapeutic Insights
Abstract
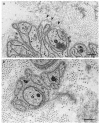
Transthyretin (TTR) amyloidosis is caused by systemic deposition of wild-type or variant amyloidogenic TTR (ATTRwt and ATTRv, respectively). ATTRwt amyloidosis has traditionally been termed senile systemic amyloidosis, while ATTRv amyloidosis has been called familial amyloid polyneuropathy. Although ATTRwt amyloidosis has classically been regarded as one of the causes of cardiomyopathy occurring in the elderly population, recent developments in diagnostic techniques have significantly expanded the concept of this disease. For example, this disease is now considered an important cause of carpal tunnel syndrome in the elderly population. The phenotypes of ATTRv amyloidosis also vary depending on the mutation and age of onset. Peripheral neuropathy usually predominates in patients from the conventional endemic foci, while cardiomyopathy or oculoleptomeningeal involvement may also become major problems in other patients. Electron microscopic studies indicate that the direct impact of amyloid fibrils on surrounding tissues leads to organ damage, whereas accumulating evidence suggests that nonfibrillar TTR, such as oligomeric TTR, is toxic, inducing neurodegeneration. Microangiopathy has been suggested to act as an initial lesion, increasing the leakage of circulating TTR. Regarding treatments, the efficacy of liver transplantation has been established for ATTRv amyloidosis patients, particularly patients with early-onset amyloidosis. Recent phase III clinical trials have shown the efficacy of TTR stabilizers, such as tafamidis and diflunisal, for both ATTRwt and ATTRv amyloidosis patients. In addition, a short interfering RNA (siRNA), patisiran, and an antisense oligonucleotide (ASO), inotersen, have been shown to be effective for ATTRv amyloidosis patients. Given their ability to significantly reduce the production of both wild-type and variant TTR in the liver, these gene-silencing drugs seem to be the optimal therapeutic option for ATTR amyloidosis. Hence, the long-term efficacy and tolerability of novel therapies, particularly siRNA and ASO, must be determined to establish an appropriate treatment program.
Keywords: Schwann cell; angiopathy; diflunisal; electron microscopy; oligomers; pathogenesis; pathology; protein misfolding disease; tafamidis; therapy.
Conflict of interest statement
H.K. reports consulting fees and travel fees from Pfizer. M.K. declares no conflicts of interest.
Figures




References
-
- Koike H., Misu K., Ikeda S., Ando Y., Nakazato M., Ando E., Yamamoto M., Hattori N., Sobue G., Study Group for Hereditary Neuropathy in Japan Type I (transthyretin Met30) familial amyloid polyneuropathy in Japan: Early- vs. late-onset form. Arch. Neurol. 2002;59:1771–1776. doi: 10.1001/archneur.59.11.1771. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

