Accessibility of Cations to the Selectivity Filter of KcsA in the Inactivated State: An Equilibrium Binding Study
- PMID: 30764559
- PMCID: PMC6387330
- DOI: 10.3390/ijms20030689
Accessibility of Cations to the Selectivity Filter of KcsA in the Inactivated State: An Equilibrium Binding Study
Abstract
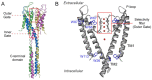
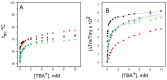
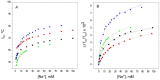
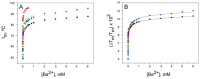
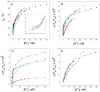
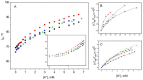
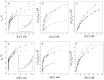
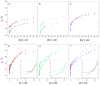
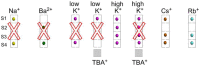
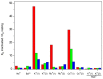
Cation binding under equilibrium conditions has been used as a tool to explore the accessibility of permeant and nonpermeant cations to the selectivity filter in three different inactivated models of the potassium channel KcsA. The results show that the stack of ion binding sites (S1 to S4) in the inactivated filter models remain accessible to cations as they are in the resting channel state. The inactivated state of the selectivity filter is therefore "resting-like" under such equilibrium conditions. Nonetheless, quantitative differences in the apparent KD's of the binding processes reveal that the affinity for the binding of permeant cations to the inactivated channel models, mainly K⁺, decreases considerably with respect to the resting channel. This is likely to cause a loss of K⁺ from the inactivated filter and consequently, to promote nonconductive conformations. The most affected site by the affinity loss seems to be S4, which is interesting because S4 is the first site to accommodate K⁺ coming from the channel vestibule when K⁺ exits the cell. Moreover, binding of the nonpermeant species, Na⁺, is not substantially affected by inactivation, meaning that the inactivated channels are also less selective for permeant versus nonpermeant cations under equilibrium conditions.
Keywords: C-type inactivation; fluorescence; ion-protein interactions; potassium channels; protein thermal stability; selectivity filter conformation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hille B. Ion Channels of Excitable Membranes. Sinauer Assoc. Inc.; Sunderland, MA, USA: 2001. p. 5.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

