At the Beginning of the End and in the Middle of the Beginning: Structure and Maintenance of Telomeric DNA Repeats and Interstitial Telomeric Sequences
- PMID: 30764567
- PMCID: PMC6410037
- DOI: 10.3390/genes10020118
At the Beginning of the End and in the Middle of the Beginning: Structure and Maintenance of Telomeric DNA Repeats and Interstitial Telomeric Sequences
Abstract
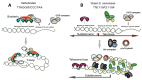
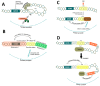
Tandem DNA repeats derived from the ancestral (TTAGGG)n run were first detected at chromosome ends of the majority of living organisms, hence the name telomeric DNA repeats. Subsequently, it has become clear that telomeric motifs are also present within chromosomes, and they were suitably called interstitial telomeric sequences (ITSs). It is well known that telomeric DNA repeats play a key role in chromosome stability, preventing end-to-end fusions and precluding the recurrent DNA loss during replication. Recent data suggest that ITSs are also important genomic elements as they confer its karyotype plasticity. In fact, ITSs appeared to be among the most unstable microsatellite sequences as they are highly length polymorphic and can trigger chromosomal fragility and gross chromosomal rearrangements. Importantly, mechanisms responsible for their instability appear to be similar to the mechanisms that maintain the length of genuine telomeres. This review compares the mechanisms of maintenance and dynamic properties of telomeric repeats and ITSs and discusses the implications of these dynamics on genome stability.
Keywords: alternative lengthening of telomeres; genome stability; microsatellites; repeat expansion; telomeres; telomeric repeats.
Conflict of interest statement
The authors declare no conflicts of interest
Figures



References
-
- Muller H.J. The remaking of chromosomes. Collect. Net. 1938;13:181–198.
-
- McClintock B. The fusion of broken ends of sister half chromatids following chromatid breakage at meiotic anaphase. Miss. Agric. Exp. Stn. Res. Bull. 1938;190:1–48.
-
- Muller H.J. Induced mutations in Drosophila. Cold Spring Harb. Symp. Quant. Biol. 1941;9:151–167. doi: 10.1101/SQB.1941.009.01.019. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

