Metabolic Changes in Spontaneously Hypertensive Rat Hearts Precede Cardiac Dysfunction and Left Ventricular Hypertrophy
- PMID: 30764689
- PMCID: PMC6405673
- DOI: 10.1161/JAHA.118.010926
Metabolic Changes in Spontaneously Hypertensive Rat Hearts Precede Cardiac Dysfunction and Left Ventricular Hypertrophy
Abstract
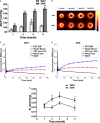
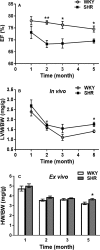
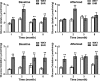
Background Sustained pressure overload leads to changes in cardiac metabolism, function, and structure. Both time course and causal relationships between these changes are not fully understood. Therefore, we studied spontaneously hypertensive rats (SHR) during early hypertension development and compared them to control Wistar Kyoto rats. Methods and Results We serially evaluated myocardial glucose uptake rates (Ki) with dynamic 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography, and ejection fraction and left ventricular mass to body weight ratios with cardiac magnetic resonance imaging in vivo, determined glucose uptake and oxidation rates in isolated perfused hearts, and analyzed metabolites, mammalian target of rapamycin activity and endoplasmic reticulum stress in dissected hearts. When compared with Wistar Kyoto rats, SHR demonstrated increased glucose uptake rates (Ki) in vivo, and reduced ejection fraction as early as 2 months of age when hypertension was established. Isolated perfused SHR hearts showed increased glucose uptake and oxidation rates starting at 1 month. Cardiac metabolite analysis at 2 months of age revealed elevated pyruvate, fatty acyl- and branched chain amino acid-derived carnitines, oxidative stress, and inflammation. Mammalian target of rapamycin activity increased in SHR beginning at 2 months. Left ventricular mass to body weight ratios and endoplasmic reticulum stress were elevated in 5 month-old SHR. Conclusions Thus, in a genetic hypertension model, chronic cardiac pressure overload promptly leads to increased myocardial glucose uptake and oxidation, and to metabolite abnormalities. These coincide with, or precede, cardiac dysfunction while left ventricular hypertrophy develops only later. Myocardial metabolic changes may thus serve as early diagnostic markers for hypertension-induced left ventricular hypertrophy.
Keywords: hypertension; hypertrophy/remodeling; metabolic imaging; myocardial metabolism; rats.
Figures

References
-
- Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population‐based study. N Engl J Med. 2006;355:260–269. - PubMed
-
- De Jong KA, Lopaschuk GD. Complex energy metabolic changes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Can J Cardiol. 2017;33:860–871. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

