The great escape: How metastases of melanoma, and other carcinomas, avoid elimination
- PMID: 30764707
- PMCID: PMC6348591
- DOI: 10.1177/1535370218820287
The great escape: How metastases of melanoma, and other carcinomas, avoid elimination
Abstract
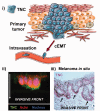
Cancers kill mainly because metastatic disease is resistant to systemic therapies. It was hoped that newer targeted and immunomodulatory interventions could overcome these issues. However, recent findings point to a generalized resistance to elimination imparted by both cancer-intrinsic and -extrinsic changes to provide survival advantages to the disseminated tumor cells. Here, we present a novel conceptual framework for the microenvironmental inputs and changes that contribute to this generalized therapeutic resistance. In addition we address the issues of experimental systems in terms of studying this phenomenon with their advantages and limitations. This is meant to spur studies into this critical aspect of tumor progression that directly leads to cancer mortality.
Keywords: Liver metastasis; hepatic niche; metastatic models; microphysiological; tumor microenvironment.
Figures



References
-
- Mieog JS, vanderHage JA, vandeVelde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg 2007; 94:1189–200 - PubMed
-
- Redden MH, Fuhrman GM. Neoadjuvant chemotherapy in the treatment of breast cancer. Surg Clin North Am 2013; 93:493–9 - PubMed
-
- Mougalian SS, Hernandez M, Lei X, Lynch S, Kuerer HM, Symmans W, Theriault RL, Fornage BD, Hsu L, Buchholz TA, Sahin AA, Hunt KK, Yang WT, Hortobagyi GN, Valero V. Ten-year outcomes of patients with breast cancer with cytologically confirmed axillary lymph node metastases and pathologic complete response after primary systemic chemotherapy. JAMA Oncol 2016; 2:508–16 - PMC - PubMed
-
- Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer 2003; 3:453–8 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

