Microscopic description of acid-base equilibrium
- PMID: 30765522
- PMCID: PMC6410861
- DOI: 10.1073/pnas.1819771116
Microscopic description of acid-base equilibrium
Abstract
Acid-base reactions are ubiquitous in nature. Understanding their mechanisms is crucial in many fields, from biochemistry to industrial catalysis. Unfortunately, experiments give only limited information without much insight into the molecular behavior. Atomistic simulations could complement experiments and shed precious light on microscopic mechanisms. The large free-energy barriers connected to proton dissociation, however, make the use of enhanced sampling methods mandatory. Here we perform an ab initio molecular dynamics (MD) simulation and enhance sampling with the help of metadynamics. This has been made possible by the introduction of descriptors or collective variables (CVs) that are based on a conceptually different outlook on acid-base equilibria. We test successfully our approach on three different aqueous solutions of acetic acid, ammonia, and bicarbonate. These are representative of acid, basic, and amphoteric behavior.
Keywords: acid–base; collective variables; enhanced sampling; metadynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
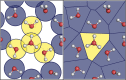
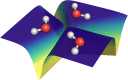
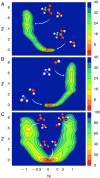
References
-
- Ho J, Coote ML. First-principles prediction of acidities in the gas and solution phase. Wiley Interdiscip Rev Comput Mol Sci. 2011;1:649–660.
-
- Elstner M, Hobza P, Frauenheim T, Suhai S, Kaxiras E. Hydrogen bonding and stacking interactions of nucleic acid base pairs: A density-functional-theory based treatment. J Chem Phys. 2001;114:5149–5155.
-
- Saracino GAA, Improta R, Barone V. Absolute pKa determination for carboxylic acids using density functional theory and the polarizable continuum model. Chem Phys Lett. 2003;373:411–415.
-
- Schüürmann G, Cossi M, Barone V, Tomasi J. Prediction of the pKa of carboxylic acids using the ab initio continuum-solvation model PCM-UAHF. J Phys Chem A. 1998;102:6706–6712.
-
- Ho J, Coote ML. A universal approach for continuum solvent pKa calculations: Are we there yet? Theor Chem Acc. 2009;125:3–21.
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

