Concentric organization of A- and B-type lamins predicts their distinct roles in the spatial organization and stability of the nuclear lamina
- PMID: 30765529
- PMCID: PMC6410836
- DOI: 10.1073/pnas.1810070116
Concentric organization of A- and B-type lamins predicts their distinct roles in the spatial organization and stability of the nuclear lamina
Abstract
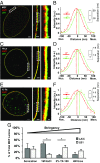
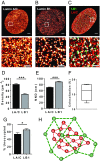
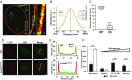
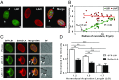
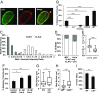
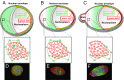
The nuclear lamina is an intermediate filament meshwork adjacent to the inner nuclear membrane (INM) that plays a critical role in maintaining nuclear shape and regulating gene expression through chromatin interactions. Studies have demonstrated that A- and B-type lamins, the filamentous proteins that make up the nuclear lamina, form independent but interacting networks. However, whether these lamin subtypes exhibit a distinct spatial organization or whether their organization has any functional consequences is unknown. Using stochastic optical reconstruction microscopy (STORM) our studies reveal that lamin B1 and lamin A/C form concentric but overlapping networks, with lamin B1 forming the outer concentric ring located adjacent to the INM. The more peripheral localization of lamin B1 is mediated by its carboxyl-terminal farnesyl group. Lamin B1 localization is also curvature- and strain-dependent, while the localization of lamin A/C is not. We also show that lamin B1's outer-facing localization stabilizes nuclear shape by restraining outward protrusions of the lamin A/C network. These two findings, that lamin B1 forms an outer concentric ring and that its localization is energy-dependent, are significant as they suggest a distinct model for the nuclear lamina-one that is able to predict its behavior and clarifies the distinct roles of individual nuclear lamin proteins and the consequences of their perturbation.
Keywords: bleb; curvature; lamin; meshwork; nucleus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

