Extreme diversification of floral volatiles within and among species of Lithophragma (Saxifragaceae)
- PMID: 30765532
- PMCID: PMC6410829
- DOI: 10.1073/pnas.1809007116
Extreme diversification of floral volatiles within and among species of Lithophragma (Saxifragaceae)
Abstract
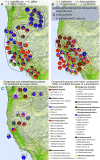
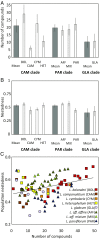
A major challenge in evolutionary biology is to understand how complex traits of multiple functions have diversified and codiversified across interacting lineages and geographic ranges. We evaluate intra- and interspecific variation in floral scent, which is a complex trait of documented importance for mutualistic and antagonistic interactions between plants, pollinators, and herbivores. We performed a large-scale, phylogenetically structured study of an entire plant genus (Lithophragma, Saxifragaceae), of which several species are coevolving with specialized pollinating floral parasites of the moth genus Greya (Prodoxidae). We sampled 94 Lithophragma populations distributed across all 12 recognized Lithophragma species and subspecies, and four populations of related saxifragaceous species. Our results reveal an unusually high diversity of floral volatiles among populations, species, and clades within the genus. Moreover, we found unexpectedly major changes at each of these levels in the biosynthetic pathways used by local populations in their floral scents. Finally, we detected significant, but variable, genus- and species-level patterns of ecological convergence in the floral scent signal, including an impact of the presence and absence of two pollinating Greya moth species. We propose that one potential key to understanding floral scent variation in this hypervariable genus is its geographically diverse interactions with the obligate specialized Greya moths and, in some species and sites, more generalized copollinators.
Keywords: floral parasitism; floral volatiles; geographic mosaic of coevolution; geographic variation; pollination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Extreme divergence in floral scent among woodland star species (Lithophragma spp.) pollinated by floral parasites.Ann Bot. 2013 Apr;111(4):539-50. doi: 10.1093/aob/mct007. Epub 2013 Jan 29. Ann Bot. 2013. PMID: 23365407 Free PMC article.
-
Nutrient availability affects floral scent much less than other floral and vegetative traits in Lithophragma bolanderi.Ann Bot. 2017 Sep 1;120(3):471-478. doi: 10.1093/aob/mcx069. Ann Bot. 2017. PMID: 28655187 Free PMC article.
-
Diversity of floral visitors to sympatric Lithophragma species differing in floral morphology.Oecologia. 2010 Jan;162(1):71-80. doi: 10.1007/s00442-009-1424-8. Epub 2009 Aug 11. Oecologia. 2010. PMID: 19669796 Free PMC article.
-
Do Plants Eavesdrop on Floral Scent Signals?Trends Plant Sci. 2016 Jan;21(1):9-15. doi: 10.1016/j.tplants.2015.09.001. Epub 2015 Oct 17. Trends Plant Sci. 2016. PMID: 26476624 Review.
-
Volatile Organic Compounds from Orchids: From Synthesis and Function to Gene Regulation.Int J Mol Sci. 2020 Feb 10;21(3):1160. doi: 10.3390/ijms21031160. Int J Mol Sci. 2020. PMID: 32050562 Free PMC article. Review.
Cited by
-
A parasitoid's dilemma between food and host resources: the role of volatiles from nectar-providing marigolds and host-infested plants attracting Aphidius platensis.Naturwissenschaften. 2021 Dec 16;109(1):9. doi: 10.1007/s00114-021-01780-8. Naturwissenschaften. 2021. PMID: 34913094
-
Diversification of terpenoid emissions proposes a geographic structure based on climate and pathogen composition in Japanese cedar.Sci Rep. 2021 Apr 15;11(1):8307. doi: 10.1038/s41598-021-87810-x. Sci Rep. 2021. PMID: 33859305 Free PMC article.
-
Intraspecific variation of scent and its impact on pollinators' preferences.AoB Plants. 2023 Jul 21;15(4):plad049. doi: 10.1093/aobpla/plad049. eCollection 2023 Jul. AoB Plants. 2023. PMID: 37560761 Free PMC article.
-
Allelic variation of terpene synthases drives terpene diversity in the wild species of the Freesia genus.Plant Physiol. 2023 Jul 3;192(3):2419-2435. doi: 10.1093/plphys/kiad172. Plant Physiol. 2023. PMID: 36932696 Free PMC article.
-
A novel O-methyltransferase Cp4MP-OMT catalyses the final step in the biosynthesis of the volatile 1,4-dimethoxybenzene in pumpkin (Cucurbita pepo) flowers.BMC Plant Biol. 2024 Apr 17;24(1):294. doi: 10.1186/s12870-024-04955-3. BMC Plant Biol. 2024. PMID: 38632532 Free PMC article.
References
-
- Kawecki TJ, Ebert D. Conceptual issues in local adaptation. Ecol Lett. 2004;7:1225–1241.
-
- Gandon S, Nuismer SL. Interactions between genetic drift, gene flow, and selection mosaics drive parasite local adaptation. Am Nat. 2009;173:212–224. - PubMed
-
- Hereford J. A quantitative survey of local adaptation and fitness trade-offs. Am Nat. 2009;173:579–588. - PubMed
-
- Thompson JN. The Geographic Mosaic of Coevolution. Univ of Chicago Press; Chicago: 2005.

