Structural insight into substrate and inhibitor discrimination by human P-glycoprotein
- PMID: 30765569
- PMCID: PMC6800160
- DOI: 10.1126/science.aav7102
Structural insight into substrate and inhibitor discrimination by human P-glycoprotein
Abstract
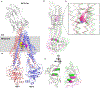
ABCB1, also known as P-glycoprotein, actively extrudes xenobiotic compounds across the plasma membrane of diverse cells, which contributes to cellular drug resistance and interferes with therapeutic drug delivery. We determined the 3.5-angstrom cryo-electron microscopy structure of substrate-bound human ABCB1 reconstituted in lipidic nanodiscs, revealing a single molecule of the chemotherapeutic compound paclitaxel (Taxol) bound in a central, occluded pocket. A second structure of inhibited, human-mouse chimeric ABCB1 revealed two molecules of zosuquidar occupying the same drug-binding pocket. Minor structural differences between substrate- and inhibitor-bound ABCB1 sites are amplified toward the nucleotide-binding domains (NBDs), revealing how the plasticity of the drug-binding site controls the dynamics of the adenosine triphosphate-hydrolyzing NBDs. Ordered cholesterol and phospholipid molecules suggest how the membrane modulates the conformational changes associated with drug binding and transport.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
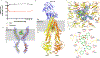
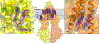
References
-
- Fromm MF, Importance of P-glycoprotein at blood-tissue barriers. Trends Pharmacol Sci 25, 423–429 (2004). - PubMed
-
- Borst P, Elferink RO, Mammalian ABC transporters in health and disease. Annu Rev Biochem 71, 537–592 (2002). - PubMed
-
- Loscher W, Potschka H, Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci 6, 591–602 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

