A Surface Plasmon Resonance-based assay to measure serum concentrations of therapeutic antibodies and anti-drug antibodies
- PMID: 30765716
- PMCID: PMC6376047
- DOI: 10.1038/s41598-018-37950-4
A Surface Plasmon Resonance-based assay to measure serum concentrations of therapeutic antibodies and anti-drug antibodies
Abstract
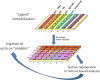
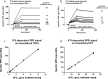
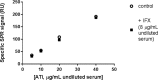
Therapeutic drug and immunogenicity monitoring (TDIM) is increasingly proposed to guide therapy with biologics, characterised by high inter-individual variability of their blood levels, to permit objective decisions for the management of non-responders and reduce unnecessary interventions with these expensive treatments. However, TDIM has not yet entered clinical practice partly because of uncertainties regarding the accuracy and precision of enzyme-linked immunosorbent assays (ELISA). Here we report the characterisation of a novel surface plasmon resonance (SPR)-based TDIM, applied to the measurement of serum concentrations of infliximab, an antibody against tumour necrosis factor α (anti-TNFα), and anti-infliximab antibodies. SPR has the obvious advantages of directly detecting and measuring serum antibodies in minutes, avoiding the long incubation/separation/washing/detection steps of the methods proposed so far, reducing complexity and variability. Moreover, drug and anti-drug antibodies can be measured simultaneously. This new method was validated for sensitivity and reproducibility, and showed cost-effectiveness over commercial ELISA kits. This method may be applied to other biotherapeutics. These data pave the way for the development of SPR-based point-of-care devices for rapid on-site analysis.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

