PDGF-BB serum levels are decreased in adult onset Pompe patients
- PMID: 30765719
- PMCID: PMC6375999
- DOI: 10.1038/s41598-018-38025-0
PDGF-BB serum levels are decreased in adult onset Pompe patients
Abstract
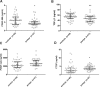
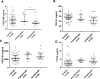
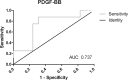
Adult onset Pompe disease is a genetic disorder characterized by slowly progressive skeletal and respiratory muscle weakness. Symptomatic patients are treated with enzymatic replacement therapy with human recombinant alfa glucosidase. Motor functional tests and spirometry are commonly used to follow patients up. However, a serological biomarker that correlates with the progression of the disease could improve follow-up. We studied serum concentrations of TGFβ, PDGF-BB, PDGF-AA and CTGF growth factors in 37 adult onset Pompe patients and 45 controls. Moreover, all patients performed several muscle function tests, conventional spirometry, and quantitative muscle MRI using 3-point Dixon. We observed a statistically significant change in the serum concentration of each growth factor in patients compared to controls. However, only PDGF-BB levels were able to differentiate between asymptomatic and symptomatic patients, suggesting its potential role in the follow-up of asymptomatic patients. Moreover, our results point to a dysregulation of muscle regeneration as an additional pathomechanism of Pompe disease.
Conflict of interest statement
The authors declare no competing interests.
Figures