Autofluorescence is a Reliable in vitro Marker of Cellular Senescence in Human Mesenchymal Stromal Cells
- PMID: 30765770
- PMCID: PMC6376004
- DOI: 10.1038/s41598-019-38546-2
Autofluorescence is a Reliable in vitro Marker of Cellular Senescence in Human Mesenchymal Stromal Cells
Abstract
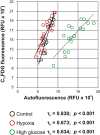
Mesenchymal stromal cells (MSC) are used in cell therapies, however cellular senescence increases heterogeneity of cell populations and leads to uncertainty in therapies' outcomes. The determination of cellular senescence is time consuming and logistically intensive. Here, we propose the use of endogenous autofluorescence as real-time quantification of cellular senescence in human MSC, based on label-free flow cytometry analysis. We correlated cell autofluorescence to senescence using senescence-associated beta-galactosidase assay (SA-β-Gal) with chromogenic (X-GAL) and fluorescent (C12FDG) substrates, gene expression of senescence markers (such as p16INK4A, p18INK4C, CCND2 and CDCA7) and telomere length. Autofluorescence was further correlated to MSC differentiation assays (adipogenesis, chondrogenesis and osteogenesis), MSC stemness markers (CD90/CD106) and cytokine secretion (IL-6 and MCP-1). Increased cell autofluorescence significantly correlated with increased SA-β-Gal signal (both X-GAL and C12FDG substrates), cell volume and cell granularity, IL-6/MCP-1 secretion and with increased p16INK4A and CCND2 gene expression. Increased cell autofluorescence was negatively associated with the expression of the CD90/CD106 markers, osteogenic and chondrogenic differentiation potentials and p18INK4C and CDCA7 gene expression. Cell autofluorescence correlated neither with telomere length nor with adipogenic differentiation potential. We conclude that autofluorescence can be used as fast and non-invasive senescence assay for comparing MSC populations under controlled culture conditions.
Conflict of interest statement
The authors declare no competing interests.
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

