Chaperone Mediated Autophagy in the Crosstalk of Neurodegenerative Diseases and Metabolic Disorders
- PMID: 30766511
- PMCID: PMC6365421
- DOI: 10.3389/fendo.2018.00778
Chaperone Mediated Autophagy in the Crosstalk of Neurodegenerative Diseases and Metabolic Disorders
Abstract
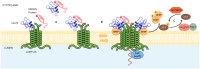
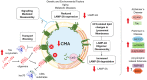
Chaperone Mediated Autophagy (CMA) is a lysosomal-dependent protein degradation pathway. At least 30% of cytosolic proteins can be degraded by this process. The two major protein players of CMA are LAMP-2A and HSC70. While LAMP-2A works as a receptor for protein substrates at the lysosomal membrane, HSC70 specifically binds protein targets and takes them for CMA degradation. Because of the broad spectrum of proteins able to be degraded by CMA, this pathway has been involved in physiological and pathological processes such as lipid and carbohydrate metabolism, and neurodegenerative diseases, respectively. Both, CMA, and the mentioned processes, are affected by aging and by inadequate nutritional habits such as a high fat diet or a high carbohydrate diet. Little is known regarding about CMA, which is considered a common regulation factor that links metabolism with neurodegenerative disorders. This review summarizes what is known about CMA, focusing on its molecular mechanism, its role in protein, lipid and carbohydrate metabolism. In addition, the review will discuss how CMA could be linked to protein, lipids and carbohydrate metabolism within neurodegenerative diseases. Furthermore, it will be discussed how aging and inadequate nutritional habits can have an impact on both CMA activity and neurodegenerative disorders.
Keywords: CMA; carbohydrates; lipids; metabolism; neurodegeneration.
Figures
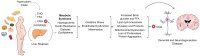
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

