CD11c+ Cells Are Gatekeepers for Lymphocyte Trafficking to Infiltrated Islets During Type 1 Diabetes
- PMID: 30766536
- PMCID: PMC6365440
- DOI: 10.3389/fimmu.2019.00099
CD11c+ Cells Are Gatekeepers for Lymphocyte Trafficking to Infiltrated Islets During Type 1 Diabetes
Abstract
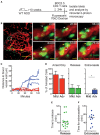
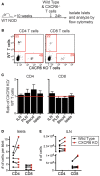
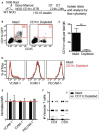
Type 1 diabetes (T1D) is a T cell mediated autoimmune disease that affects more than 19 million people with incidence increasing rapidly worldwide. For T cells to effectively drive T1D, they must first traffic to the islets and extravasate through the islet vasculature. Understanding the cues that lead to T cell entry into inflamed islets is important because diagnosed T1D patients already have established immune infiltration of their islets. Here we show that CD11c+ cells are a key mediator of T cell trafficking to infiltrated islets in non-obese diabetic (NOD) mice. Using intravital 2-photon islet imaging we show that T cell extravasation into the islets is an extended process, with T cells arresting in the islet vasculature in close proximity to perivascular CD11c+ cells. Antigen is not required for T cell trafficking to infiltrated islets, but T cell chemokine receptor signaling is necessary. Using RNAseq, we show that islet CD11c+ cells express over 20 different chemokines that bind chemokine receptors expressed on islet T cells. One highly expressed chemokine-receptor pair is CXCL16-CXCR6. However, NOD. CXCR6-/- mice progressed normally to T1D and CXCR6 deficient T cells trafficked normally to the islets. Even with CXCR3 and CXCR6 dual deficiency, T cells trafficked to infiltrated islets. These data reinforce that chemokine receptor signaling is highly redundant for T cell trafficking to inflamed islets. Importantly, depletion of CD11c+ cells strongly inhibited T cell trafficking to infiltrated islets of NOD mice. We suggest that targeted depletion of CD11c+ cells associated with the islet vasculature may yield a therapeutic target to inhibit T cell trafficking to inflamed islets to prevent progression of T1D.
Keywords: CD11c+ cells; T cell; chemokine; extravasation; lymphocyte trafficking; mononuclear phagocyte cells; type 1 diabetes.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

