Osteoarthritic Synovial Fluid Modulates Cell Phenotype and Metabolic Behavior In Vitro
- PMID: 30766606
- PMCID: PMC6350599
- DOI: 10.1155/2019/8169172
Osteoarthritic Synovial Fluid Modulates Cell Phenotype and Metabolic Behavior In Vitro
Abstract
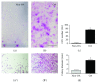
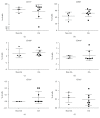
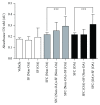
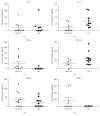
Synovial fluid holds a population of mesenchymal stem cells (MSC) that could be used for clinical treatment. Our goal was to characterize the inflammatory and metabolomic profile of the synovial fluid from osteoarthritic patients and to identify its modulatory effect on synovial fluid cells. Synovial fluid was collected from non-OA and OA patients, which was centrifuged to isolate cells. Cells were cultured for 21 days, characterized with specific markers for MSC, and exposed to a specific cocktail to induce chondrogenic, osteogenic, and adipogenic differentiation. Then, we performed a MTT assay exposing SF cells from non-OA and OA patients to a medium containing non-OA and OA synovial fluid. Synovial fluid from non-OA and OA patients was submitted to ELISA to evaluate BMP-2, BMP-4, IL-6, IL-10, TNF-α, and TGF-β1 concentrations and to a metabolomic evaluation using 1H-NMR. Synovial fluid cells presented spindle-shaped morphology in vitro. Samples from OA patients formed a higher number of colonies than the ones from non-OA patients. After 21 days, the colony-forming cells from OA patients differentiated into the three mesenchymal cell lineages, under the appropriated induction protocols. Synovial fluid cells increased its metabolic activity after being exposed to the OA synovial fluid. ELISA assay showed that OA synovial fluid samples presented higher concentration of IL-10 and TGF-β1 than the non-OA, while the NMR showed that OA synovial fluid presents higher concentrations of glucose and glycerol. In conclusion, SFC activity is modulated by OA synovial fluid, which presents higher concentration of IL-10, TGF-β, glycerol, and glucose.
Figures


References
-
- Safran M. R., Kim H., Zaffagnini S. The use of scaffolds in the management of articular injury. JAAOS. 2008;16(6):306–311. - PubMed
LinkOut - more resources
Full Text Sources

