Impaired Hepatic Phosphatidylcholine Synthesis Leads to Cholestasis in Mice Challenged With a High-Fat Diet
- PMID: 30766963
- PMCID: PMC6357837
- DOI: 10.1002/hep4.1302
Impaired Hepatic Phosphatidylcholine Synthesis Leads to Cholestasis in Mice Challenged With a High-Fat Diet
Abstract
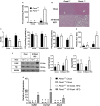
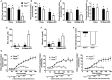
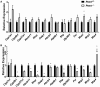
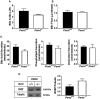
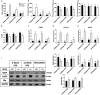
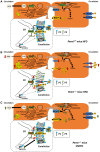
Phosphatidylethanolamine N-methyltransferase (PEMT) is a hepatic integral membrane protein localized to the endoplasmic reticulum (ER). PEMT catalyzes approximately 30% of hepatic phosphatidylcholine (PC) biosynthesis. Pemt-/- mice fed a high-fat diet (HFD) develop steatohepatitis. Interestingly, portions of the ER located close to the canaliculus are enriched in PEMT. Phospholipid balance and asymmetrical distribution by adenosine triphosphatase phospholipid transporting 8B1 (ATP8B1) on the canalicular membrane is required for membrane integrity and biliary processes. We hypothesized that PEMT is an important supplier of PC to the canaliculus and that PEMT activity is critical for the maintenance of canalicular membrane integrity and bile formation following HFD feeding when there is an increase in overall hepatic PC demand. Pemt+/+ and Pemt-/- mice were fed a chow diet, an HFD, or a choline-supplemented HFD. Plasma and hepatic indices of liver function and parameters of bile formation were determined. Pemt-/- mice developed cholestasis, i.e, elevated plasma bile acid (BA) concentrations and decreased biliary secretion rates of BAs and PC, during HFD feeding. The maximal BA secretory rate was reduced more than 70% in HFD-fed Pemt-/- mice. Hepatic ABCB11/bile salt export protein, responsible for BA secretion, was decreased in Pemt-/- mice and appeared to be retained intracellularly. Canalicular membranes of HFD-fed Pemt-/- mice contained fewer invaginations and displayed a smaller surface area than Pemt+/+ mice. Choline supplementation (CS) prevented and reversed the development of HFD-induced cholestasis. Conclusion: We propose that hepatic PC availability is critical for bile formation. Dietary CS might be a potential noninvasive therapy for a specific subset of patients with cholestasis.
Figures

References
-
- DeLong CJ, Shen YJ, Thomas MJ, Cui Z. Molecular distinction of phosphatidylcholine synthesis between the CDP‐choline pathway and phosphatidylethanolamine methylation pathway. J Biol Chem 1999;274:29683‐29688. - PubMed
-
- Noga AA, Zhao Y, Vance DE. An unexpected requirement for phosphatidylethanolamine N‐methyltransferase in the secretion of very low density lipoproteins. J Biol Chem 2002;277:42358‐42365. - PubMed
-
- Ling J, Chaba T, Zhu LF, Jacobs RL, Vance DE. Hepatic ratio of phosphatidylcholine to phosphatidylethanolamine predicts survival after partial hepatectomy in mice. Hepatology 2012;55:1094‐1102. - PubMed
-
- Li Z, Agellon LB, Allen TM, Umeda M, Jewell L, Mason A, et al. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab 2006;3:321‐331. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

