Handling interoccasion variability in model-based dose individualization using therapeutic drug monitoring data
- PMID: 30767254
- PMCID: PMC6533430
- DOI: 10.1111/bcp.13901
Handling interoccasion variability in model-based dose individualization using therapeutic drug monitoring data
Abstract
Aims: This study aims to assess approaches to handle interoccasion variability (IOV) in a model-based therapeutic drug monitoring (TDM) context, using a population pharmacokinetic model of coagulation factor VIII as example.
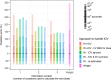
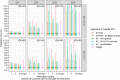
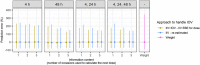
Methods: We assessed 5 model-based TDM approaches: empirical Bayes estimates (EBEs) from a model including IOV, with individualized doses calculated based on individual parameters either (i) including or (ii) excluding variability related to IOV; and EBEs from a model excluding IOV by (iii) setting IOV to zero, (iv) summing variances of interindividual variability (IIV) and IOV into a single IIV term, or (v) re-estimating the model without IOV. The impact of varying IOV magnitudes (0-50%) and number of occasions/observations was explored. The approaches were compared with conventional weight-based dosing. Predictive performance was assessed with the prediction error percentiles.
Results: When IOV was lower than IIV, the accuracy was good for all approaches (50th percentile of the prediction error [P50] <7.4%), but the precision varied substantially between IOV magnitudes (P97.5 61-528%). Approach (ii) was the most precise forecasting method across a wide range of scenarios, particularly in case of sparse sampling or high magnitudes of IOV. Weight-based dosing led to less precise predictions than the model-based TDM approaches in most scenarios.
Conclusions: Based on the studied scenarios and theoretical expectations, the best approach to handle IOV in model-based dose individualization is to include IOV in the generation of the EBEs but exclude the portion of unexplained variability related to IOV in the individual parameters used to calculate the future dose.
Keywords: NONMEM; pharmacokinetics; population analysis; therapeutic drug monitoring.
© 2019 The British Pharmacological Society.
Conflict of interest statement
There are no competing interests to declare.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

