A walk through tau therapeutic strategies
- PMID: 30767766
- PMCID: PMC6376692
- DOI: 10.1186/s40478-019-0664-z
A walk through tau therapeutic strategies
Abstract
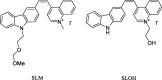
Tau neuronal and glial pathologies drive the clinical presentation of Alzheimer's disease and related human tauopathies. There is a growing body of evidence indicating that pathological tau species can travel from cell to cell and spread the pathology through the brain. Throughout the last decade, physiological and pathological tau have become attractive targets for AD therapies. Several therapeutic approaches have been proposed, including the inhibition of protein kinases or protein-3-O-(N-acetyl-beta-D-glucosaminyl)-L-serine/threonine Nacetylglucosaminyl hydrolase, the inhibition of tau aggregation, active and passive immunotherapies, and tau silencing by antisense oligonucleotides. New tau therapeutics, across the board, have demonstrated the ability to prevent or reduce tau lesions and improve either cognitive or motor impairment in a variety of animal models developing neurofibrillary pathology. The most advanced strategy for the treatment of human tauopathies remains immunotherapy, which has already reached the clinical stage of drug development. Tau vaccines or humanised antibodies target a variety of tau species either in the intracellular or extracellular spaces. Some of them recognise the amino-terminus or carboxy-terminus, while others display binding abilities to the proline-rich area or microtubule binding domains. The main therapeutic foci in existing clinical trials are on Alzheimer's disease, progressive supranuclear palsy and non-fluent primary progressive aphasia. Tau therapy offers a new hope for the treatment of many fatal brain disorders. First efficacy data from clinical trials will be available by the end of this decade.
Keywords: Aggregation; Alzheimer’s disease; Immunotherapy; PET imaging; Tau vaccines; Tauopathies; Therapeutic interventions.
Conflict of interest statement
Competing interests
SJ, RS, EK and NZ are Axon Neuroscience R&D Services employees. LB received research grants from UCB Biopharma. Other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Agadjanyan MG, Zagorski K, Petrushina I, Davtyan H, Kazarian K, Antonenko M, Davis J, Bon C, Blurton-Jones M, Cribbs DH, Ghochikyan A. Humanized monoclonal antibody armanezumab specific to N-terminus of pathological tau: characterization and therapeutic potency. Mol Neurodegener. 2017;12:33. doi: 10.1186/s13024-017-0172-1. - DOI - PMC - PubMed
-
- Ahmed T, Blum D, Burnouf S, Demeyer D, Buee-Scherrer V, D'Hooge R, Buee L, Balschun D (2015) Rescue of impaired late-phase long-term depression in a tau transgenic mouse model. Neurobiol Aging 36:730–739. 10.1016/j.neurobiolaging.2014.09.015 - PubMed

