Ovarian Metabolism of an Environmentally Relevant Phthalate Mixture
- PMID: 30768133
- PMCID: PMC6484896
- DOI: 10.1093/toxsci/kfz047
Ovarian Metabolism of an Environmentally Relevant Phthalate Mixture
Abstract
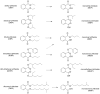
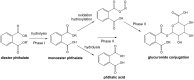
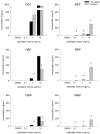
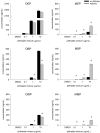
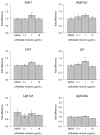
Phthalates are synthetic chemicals with widespread human exposure due to their use as additives in consumer products. Phthalate diesters are hydrolyzed in the environment and in the body to monoesters that may be more toxic than the parent compounds. This study tested the hypothesis that adult mouse antral follicles, but not neonatal ovaries, are able to metabolize an environmentally relevant mixture of phthalates. Whole neonatal ovaries and isolated adult antral follicles from CD-1 mice were cultured in media treated with vehicle control or 0.1-10 µg/ml of a mixture composed of 35% diethyl phthalate (DEP), 21% di(2-ethylhexyl) phthalate (DEHP), 15% dibutyl phthalate (DBP), 15% diisononyl phthalate (DiNP), 8% diisobutyl phthalate (DiBP), and 5% benzylbutyl phthalate (BzBP). After 4 days of culture, media were subjected to high-performance liquid chromatography tandem mass spectrometry to measure the amounts of diester phthalates and monoester metabolites. Ovaries and follicles were collected to measure the gene and protein expression of the enzymes required for phthalate metabolism. Monoester metabolites for all phthalates except DiNP were detected in the media for both culture types at most doses. The long-chain phthalates (BzBP, DEHP, and DiNP) were metabolized less than the short-chain phthalates (DEP, DBP, and DiBP) compared with respective controls. Expression of metabolizing enzymes was observed for all treatment groups in both culture types. These data indicate that mouse ovaries are capable of metabolizing low doses of phthalates and suggest that metabolic capacity differs for follicles at different stages of development.
Keywords: follicles; metabolism; ovary; phthalates.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures





References
-
- Adibi J. J., Zhao Y., Zhan L. V., Kapidzic M., Larocque N., Koistinen H., Huhtaniemi I. T., Stenman U. H. (2017). An investigation of the single and combined phthalate metabolite effects on human chorionic gonadotropin expression in placental cells. Environ. Health Perspect. 125, 1–12. - PMC - PubMed
-
- Albert O., Jegou B. (2014). A critical assessment of the endocrine susceptibility of the human testis to phthalates from fetal life to adulthood. Hum. Reprod. Update 20, 231–249. - PubMed
-
- Albro P. W., Thomas R. O. (1973). Enzymatic hydrolysis of di-(2-ethylhexyl) phthalate by lipases. Biochim. Biophys. Acta 360, 380–390. - PubMed
-
- Calafat A. M., Needham L. L., Silva M. J., Lambert G. (2004). Exposure to di-(2-ethylhexyl) phthalate among premature neonates in a neonatal intensive care unit. Pediatrics 113, e429.. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

