Exposure to Cold Unmasks Potential Biomarkers of Fibromyalgia Syndrome Reflecting Insufficient Sympathetic Responses to Stress
- PMID: 30768436
- PMCID: PMC6450706
- DOI: 10.1097/AJP.0000000000000695
Exposure to Cold Unmasks Potential Biomarkers of Fibromyalgia Syndrome Reflecting Insufficient Sympathetic Responses to Stress
Abstract
Objectives: Fibromyalgia syndrome (FMS) is a chronically painful condition whose symptoms are widely reported to be exacerbated by stress. We hypothesized that female patients with FMS differ from pain-free female controls in their sympathetic responses, a fact that may unmask important biomarkers and factors that contribute to the etiology of FMS.
Materials and methods: In a pilot study, blood pressure (BP), skin temperature, thermogenic activity, circulating glucose, and pain sensitivity of 13 individuals with FMS and 11 controls at room temperature (24°C) were compared with that after exposure to cold (19°C).
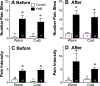
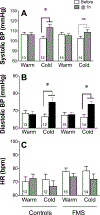
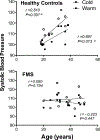
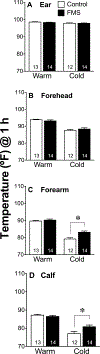
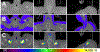
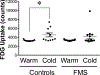
Results: When measured at 24°C, BP, skin temperature, blood glucose, and brown adipose tissue (BAT) activity, measured using F-fluorodeoxyglucose positron-emission tomography/computed tomography, did not differ between controls and individuals with FMS. However, after cold exposure (19°C), BP and BAT activity increased in controls but not in individuals with FMS; skin temperature on the calf and arm decreased in controls more than in individiuals with FMS; and circulating glucose was lower in individiuals with FMS than in controls. Pain sensitivity did not change during the testing interval in response to cold.
Discussion: The convergence of the effect of cold on 4 relatively simple measures of thermogenic, cardiovascular, and metabolic activity, each regulated by sympathetic activity, strongly indicate that individuals with FMS have impaired sympathetic responses to stress that are observable and highly significant even when measured in extraordinarily small sample populations. If insufficient sympathetic responses to stress are linked to FMS, stress may unmask and maximize these potential clinical biomarkers of FMS and be related to its etiology.
Figures

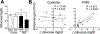
References
-
- Queiroz LP. Worldwide epidemiology of fibromyalgia. Current pain and headache reports 2013;17:356. - PubMed
-
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P and et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990;33:160–72. - PubMed
-
- Fischer S, Doerr JM, Strahler J, Mewes R, Thieme K and Nater UM. Stress exacerbates pain in the everyday lives of women with fibromyalgia syndrome--The role of cortisol and alpha-amylase. Psychoneuroendocrinology 2016;63:68–77. - PubMed
-
- D’Arcy Y, Kraus S, Clair A and Kiley D. Fibromyalgia: Timely diagnosis and treatment options. Nurse Pract 2016;41:37–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

