Fast near-whole-brain imaging in adult Drosophila during responses to stimuli and behavior
- PMID: 30768592
- PMCID: PMC6395010
- DOI: 10.1371/journal.pbio.2006732
Fast near-whole-brain imaging in adult Drosophila during responses to stimuli and behavior
Abstract
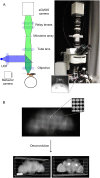
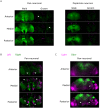
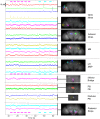
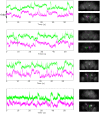
Whole-brain recordings give us a global perspective of the brain in action. In this study, we describe a method using light field microscopy to record near-whole brain calcium and voltage activity at high speed in behaving adult flies. We first obtained global activity maps for various stimuli and behaviors. Notably, we found that brain activity increased on a global scale when the fly walked but not when it groomed. This global increase with walking was particularly strong in dopamine neurons. Second, we extracted maps of spatially distinct sources of activity as well as their time series using principal component analysis and independent component analysis. The characteristic shapes in the maps matched the anatomy of subneuropil regions and, in some cases, a specific neuron type. Brain structures that responded to light and odor were consistent with previous reports, confirming the new technique's validity. We also observed previously uncharacterized behavior-related activity as well as patterns of spontaneous voltage activity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Lemon WC, Pulver SR, Hockendorf B, McDole K, Branson K, Freeman J, et al. Whole-central nervous system functional imaging in larval Drosophila. Nat Commun. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved.; 2015;6 Available: 10.1038/ncomms8924 - DOI - PMC - PubMed
-
- Levoy M, Ng R, Adams A, Footer M, Horowitz M. Light field microscopy. ACM Trans Graph. 2006;25: 924 10.1145/1141911.1141976 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

