Photoredox-Catalyzed Site-Selective α-C(sp3 )-H Alkylation of Primary Amine Derivatives
- PMID: 30768740
- PMCID: PMC6442930
- DOI: 10.1002/anie.201812227
Photoredox-Catalyzed Site-Selective α-C(sp3 )-H Alkylation of Primary Amine Derivatives
Abstract
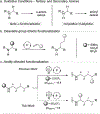
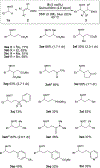
The synthetic utility of tertiary amines to oxidatively generate α-amino radicals is well established, however, primary amines remain challenging because of competitive side reactions. This report describes the site-selective α-functionalization of primary amine derivatives through the generation of α-amino radical intermediates. Employing visible-light photoredox catalysis, primary sulfonamides are coupled with electron-deficient alkenes to efficiently and mildly construct C-C bonds. Interestingly, a divergence between intermolecular hydrogen-atom transfer (HAT) catalysis and intramolecular [1,5] HAT was observed through precise manipulation of the protecting group. This dichotomy was leveraged to achieve excellent α/δ site-selectivity.
Keywords: amines; photochemistry; radicals; reaction mechanisms; synthetic methods.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
-
- McNally A, Prier K, MacMillan DWC, Science 2011, 334, 1114–1117 - PMC - PubMed
- Kohls P, Jadhav D, Pandey G, Rieser O, Org. Lett. 2012, 14, 672–675. - PubMed
- Miyake Y, Nakajima K, Nishibayashi Y, J. Am. Chem. Soc. 2012, 134, 3338–3341. - PubMed
- Nakajima K, Miyake Y, Nishibayashi Y, Acc. Chem. Res. 2016, 49, 1946–1956. - PubMed
- Prier CK, Rankic DA, MacMillan DWC, Chem. Rev. 2013, 113, 5322–5363. - PMC - PubMed
- Beatty JW, Stephenson CRJ, Acc. Chem. Res. 2015, 48, 1474–1484. - PMC - PubMed
-
- Dinnocenzo JP, Banach TE, J. Am. Chem. Soc. 1989, 111, 8646–8653.
- Zhang X, Yeh S-R, Hong S, Freccero M, Albini A, Falvey DE, Mariano PS, J. Am. Chem. Soc. 1994, 116, 4211–4220.
-
- Lewis FD, Zebrowski BE, Correa PE, J. Am. Chem. Soc. 1984, 106, 187–193.
- Lewis FD, Correa PE, J. Am. Chem. Soc. 1984, 106, 194–198.
- Lewis FD, Reddy GD, Schneider S, Gahr M, J. Am. Chem. Soc. 1989, 111, 6465–6466.
- Das S, Dileep Kumar JS, Shivaramayya K, George MV, J. Chem. Soc, Perkin Trans. 1, 1995, 1797–1799.
- Cookson RC, Costa S.M. De B., Hudec J, J. Chem. Soc. D: Chem. Commun., 1969, 13, 753–754.
-
- Yoon UC, Mariano PS, Acc. Chem. Res. 1992, 25, 233–240.
- Cho DW, Yoon UC, Acc. Chem. Res. 2011, 44, 204–215. - PubMed
- Miyake Y, Ashida Y, Nakajima K, Nishibayashi Y, Chem. Commun. 2012, 48, 6966–6968. - PubMed
- Nakajima K, Kitagawa M, Ashida Y, Miyake Y, Y. Nishibayashi Y. Chem. Commun. 2014, 50, 8900–8903. - PubMed
- Ruiz Espelt L, McPherson IS, Wiensch EM, Yoon TP, J. Am. Chem. Soc. 2015, 137, 2452–2455. - PMC - PubMed
- Lenhart D, Bauer A, Pothig A, Bach T, Chem. -Eur. J. 2016, 22, 6519–6523. - PubMed
- Chu L, Ohta C, Zuo Z, MacMillan DWC, J. Am. Chem. Soc. 2014, 136, 10886–10889. - PMC - PubMed
- Yasu Y, Koike T, Akita M, Adv. Synth. Catal. 2012, 354, 3414–3420.
- Miyazawa K, Koike T, Akita M, Adv. Synth. Catal. 2014, 356, 2749–2755.
- Fan L, Jia J, Hou H, Lefebvre Q, Rueping M, Chem. Eur. J. 2016, 22, 16437–16440. - PubMed
- Zuo Z, Cong H, Li W, Choi J, Fu GC, MacMillan DWC, J. Am. Chem. Soc. 2016, 138, 1832–1835. - PMC - PubMed
-
- Le C, Liang Y, Evans RW, Li X, MacMillan DWC, Nature. 2017, 547, 79–83. - PMC - PubMed
- Shaw MH, Shurtleff VW, Terrett JA, Cuthbertson JD, MacMillan DWC, Science 2016, 352, 1304–1308. - PMC - PubMed
- Alternately, Nicewicz and coworkers recently described the formation of α-amido alkyl radicals from photoredox catalyzed direct oxidation of the tertiary amides; see: McManus JB, Pnushka NPR, Nicewicz DA, J. Am. Chem. Soc. 2018, 140,9056–9060. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

