Supramolecular Hybrid Material Based on Engineering Porphyrin Hosts for an Efficient Elimination of Lead(II) from Aquatic Medium
- PMID: 30769770
- PMCID: PMC6412391
- DOI: 10.3390/molecules24040669
Supramolecular Hybrid Material Based on Engineering Porphyrin Hosts for an Efficient Elimination of Lead(II) from Aquatic Medium
Abstract
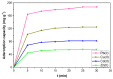
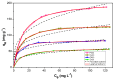
Porphyrins show great promise for future purification demands. This is largely due to their unique features as host binding molecules that can be modified at the synthetic level, and largely improved by their incorporation into inorganic based materials. In this study, we assessed the efficacy of a hybrid material obtained from the immobilization of 5,10,15,20-tetrakis(pentafluorophenyl)-porphyrin on silica surface to remove Pb(II), Cu(II), Cd(II), and Zn(II) ions from water. The new organic-inorganic hybrid adsorbent was fully characterized by adequate techniques and the results show that the hybrid exhibits good chemical and thermal stability. From batch assays, it was evaluated how the efficacy of the hybrid was affected by the pH, contact time, initial metal concentration, and temperature. The adsorption kinetic and isotherms showed to fit the recent developed fractal-like pseudo-second-order model and Langmuir⁻Freundlich model respectively. The highest adsorption capacities for Pb(II), Cu(II), Cd(II), and Zn(II) ions were 187.36, 125.17, 82.45, and 56.23 mg g-1, respectively, at pH 6.0 and 25 °C. This study also shows that metal cations from real river water samples can be efficient removed in the presence of the new adsorbent material.
Keywords: adsorption; heavy metal cation; hybrid material; lead; porphyrinoids; selectivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Bernard A. Cadmium and its adverse effects on human health. Indian J. Med. Res. 2008;128:557. - PubMed
-
- Hajdu I., Bodnár M., Csikós Z., Wei S., Daróczi L., Kovács B., Győri Z., Tamás J., Borbély J. Combined nano-membrane technology for removal of lead ions. J. Memb. Sci. 2012;409:44–53. doi: 10.1016/j.memsci.2012.03.011. - DOI
-
- Kumar R., Kumar M., Ahmad R., Barakat M. l-Methionine modified Dowex-50 ion-exchanger of reduced size for the separation and removal of Cu (II) and Ni (II) from aqueous solution. Chem. Eng. J. 2013;218:32–38. doi: 10.1016/j.cej.2012.12.029. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

