HM-Chromanone Isolated from Portulaca oleracea L. Protects INS-1 Pancreatic β Cells against Glucotoxicity-Induced Apoptosis
- PMID: 30769910
- PMCID: PMC6412778
- DOI: 10.3390/nu11020404
HM-Chromanone Isolated from Portulaca oleracea L. Protects INS-1 Pancreatic β Cells against Glucotoxicity-Induced Apoptosis
Abstract
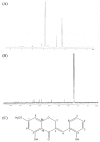
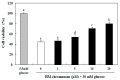
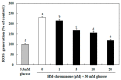
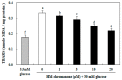
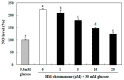
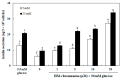
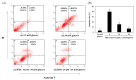
In this study, we investigated whether (E)-5-hydroxy-7-methoxy-3-(2'-hydroxybenzyl)-4-chromanone, a homoisoflavonoid compound isolated from Portulaca oleracea L., protects INS-1 pancreatic β cells against glucotoxicity-induced apoptosis. Treatment with high glucose (30 mM) induced apoptosis in INS-1 pancreatic β cells; however, the level of cell viability was significantly increased by treatment with (E)-5-hydroxy-7-methoxy-3-(2'-hydroxybenzyl)-4-chromanone. Treatment with 10⁻20 µM of (E)-5-hydroxy-7-methoxy-3-(2'-hydroxybenzyl)-4-chromanone dose-dependently increased cell viability and significantly decreased the intracellular level of reactive oxygen species (ROS), thiobarbituric acid reactive substances (TBARS), and nitric oxide levels in INS-1 pancreatic β cells pretreated with high glucose. These effects were associated with increased anti-apoptotic Bcl-2 protein expression, while reducing pro-apoptotic Bax, cytochrome C, and caspase 9 protein expression. Treatment with (E)-5-hydroxy-7-methoxy-3-(2'-hydroxybenzyl)-4-chromanone reduced the apoptosis previously induced by high-level glucose-treatment, according to annexin V/propidium iodide staining. These results demonstrate that (E)-5-hydroxy-7-methoxy-3-(2'-hydroxybenzyl)-4-chromanone may be useful as a potential therapeutic agent to protect INS-1 pancreatic β cells against high glucose-induced apoptosis.
Keywords: (E)-5-hydroxy-7-methoxy-3-(2′-hydroxybenzyl)-4-chromanone; INS-1 pancreatic β cell; Portulaca oleracea; glucotoxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sung Y.A., Hong Y.S. Mechanism of the insulin secretory defect by chronically elevated glucose levels in pancreatic islets: Depletion of insulin content due to hyperstimulation by glucose. J. Kor. Diabetes Asso. 2000;24:1–9.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

