Thyroid hormone receptor function in maturing ovine cardiomyocytes
- PMID: 30770568
- PMCID: PMC6462488
- DOI: 10.1113/JP276874
Thyroid hormone receptor function in maturing ovine cardiomyocytes
Abstract
Key points: Plasma thyroid hormone (tri-iodo-l-thyronine; T3 ) concentrations rise near the end of gestation and is known to inhibit proliferation and stimulate maturation of cardiomyocytes before birth. Thyroid hormone receptors are required for the action of thyroid hormone in fetal cardiomyocytes. Loss of thyroid hormone receptor (TR)α1 abolishes T3 signalling via extracellular signal-related kinase and Akt in fetal cardiomyocytes. The expression of TRα1 and TRβ1 in ovine fetal myocardium increases with age, although TRα1 levels always remain higher than those of TRβ1. Near term fetal cardiac myocytes are more sensitive than younger myocytes to thyroid receptor blockade by antagonist, NH3, and to the effects of TRα1/α2 short interfering RNA. Although T3 is known to abrogate ovine cardiomyocyte proliferation stimulated by insulin-like growth factor 1, this effect is mediated via the genomic action of thyroid hormone receptors, with little evidence for non-genomic mechanisms.
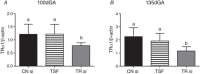
Abstract: We have previously shown that the late-term rise in tri-iodo-l-thyronine (T3 ) in fetal sheep leads to the inhibition of proliferation and promotion of maturation in cardiomyocytes. The present study was designed to determine whether these T3 -induced changes are mediated via thyroid hormone receptors (TRs) or by non-genomic mechanisms. Fetal cardiomyocytes were isolated from 102 ± 3 and 135 ± 1 days of gestational age (dGA) sheep (n = 7 per age; term ∼145 dGA). Cells were treated with T3 (1.5 nm), insulin-like growth factor (IGF)-1 (1 μg mL-1 ) or a combination in the presence of TR antagonist NH3 (100 nm) or following short interfering RNA (siRNA) knockdown of TRα1/α2. Proliferation was quantified by 5-bromo-2'-deoxyuridine (BrdU) uptake (10 μm). Western blots measured protein levels of extracellular signal-related kinase (ERK), Akt, TRα1/β1 and p21. Age specific levels of TRα1/β1 were measured in normal hearts from fetuses [95 dGA (n = 8), 135 dGA (n = 7)], neonates (n = 8) and adult ewes (n = 7). TRα1 protein levels were consistently >50% more than TRβ1 at each gestational age (P < 0.05). T3 reduced IGF-1 stimulated proliferation by ∼50% in 100 dGA and by ∼75% in 135 dGA cardiomyocytes (P < 0.05). NH3 blocked the T3 + IGF-1 reduction of BrdU uptake without altering the phosphorylation of ERK or Akt at both ages. NH3 did not suppress T3 -induced p21 expression in 100 dGA cardiomyocytes in 135 dGA cardiomyocytes, NH3 alone reduced BrdU uptake (-28%, P < 0.05), as well as T3 -induced p21 (-75%, P < 0.05). In both ages, siRNA knockdown of TRα1/α2 blocked the T3 + IGF-1 reduction of BrdU uptake and dramatically reduced ERK and Akt signalling in 135 dGA cardiomyocytes. In conclusion, TRs are required for normal proliferation and T3 signalling in fetal ovine cardiomyocytes, with the sensitivity to TR blockade being age-dependent.
Keywords: Cardiomyocyte proliferation; Fetal; NH3; Thyroid hormone.
© 2019 The Authors. The Journal of Physiology © 2019 The Physiological Society.
Figures








References
-
- Barbera A, Giraud GD, Reller MD, Maylie J, Morton MJ & Thornburg KL (2000). Right ventricular systolic pressure load alters myocyte maturation in fetal sheep. Am J Physiol Regul Integr Comp Physiol 279, R1157–R1164. - PubMed
-
- Bensley JG, Stacy VK, De Matteo R, Harding R & Black MJ (2010). Cardiac remodelling as a result of pre‐term birth: implications for future cardiovascular disease. Eur Heart J 31, 2058–2066. - PubMed
-
- Bergh JJ, Lin HY, Lansing L, Mohamed SN, Davis FB, Mousa S & Davis PJ (2005). Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen‐activated protein kinase and induction of angiogenesis. Endocrinology 146, 2864–2871. - PubMed
-
- Burrell JH, Boyn AM, Kumarasamy V, Hsieh A, Head SI & Lumbers ER (2003). Growth and maturation of cardiac myocytes in fetal sheep in the second half of gestation. Anat Rec A Discov Mol Cell Evol Biol 274, 952–961. - PubMed
-
- Burton PB, Raff MC, Kerr P, Yacoub MH & Barton PJ (1999). An intrinsic timer that controls cell‐cycle withdrawal in cultured cardiac myocytes. Dev Biol 216, 659–670. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

