Functional connectivity associated with tau levels in ageing, Alzheimer's, and small vessel disease
- PMID: 30770704
- PMCID: PMC6439332
- DOI: 10.1093/brain/awz026
Functional connectivity associated with tau levels in ageing, Alzheimer's, and small vessel disease
Abstract
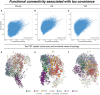
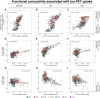
In Alzheimer's disease, tau pathology spreads hierarchically from the inferior temporal lobe throughout the cortex, ensuing cognitive decline and dementia. Similarly, circumscribed patterns of pathological tau have been observed in normal ageing and small vessel disease, suggesting a spatially ordered distribution of tau pathology across normal ageing and different diseases. In vitro findings suggest that pathological tau may spread 'prion-like' across neuronal connections in an activity-dependent manner. Supporting this notion, functional brain networks show a spatial correspondence to tau deposition patterns. However, it remains unclear whether higher network-connectivity facilitates tau propagation. To address this, we included 55 normal aged elderly (i.e. cognitively normal, amyloid-negative), 50 Alzheimer's disease patients (i.e. amyloid-positive) covering the preclinical to dementia spectrum, as well as 36 patients with pure (i.e. amyloid-negative) vascular cognitive impairment due to small vessel disease. All subjects were assessed with AV1451 tau-PET and resting-state functional MRI. Within each group, we computed atlas-based resting-state functional MRI functional connectivity across 400 regions of interest covering the entire neocortex. Using the same atlas, we also assessed within each group the covariance of tau-PET levels among the 400 regions of interest. We found that higher resting-state functional MRI assessed functional connectivity between any given region of interest pair was associated with higher covariance in tau-PET binding in corresponding regions of interest. This result was consistently found in normal ageing, Alzheimer's disease and vascular cognitive impairment. In particular, inferior temporal tau-hotspots, as defined by highest tau-PET uptake, showed high predictive value of tau-PET levels in functionally closely connected regions of interest. These associations between functional connectivity and tau-PET uptake were detected regardless of presence of dementia symptoms (mild cognitive impairment or dementia), amyloid deposition (as assessed by amyloid-PET) or small vessel disease. Our findings suggest that higher functional connectivity between brain regions is associated with shared tau-levels, supporting the view of prion-like tau spreading facilitated by neural activity.
Keywords: Alzheimer’s disease; functional connectivity; resting-state functional MRI; tau-PET; tau-spreading.
© The Author(s) (2019). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Abhinav K, Yeh FC, Pathak S, Suski V, Lacomis D, Friedlander RM, et al.Advanced diffusion MRI fiber tracking in neurosurgical and neurodegenerative disorders and neuroanatomical studies: a review. Biochim Biophys Acta 2014; 1842: 2286–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

