Neurotensin and its receptors mediate neuroendocrine transdifferentiation in prostate cancer
- PMID: 30770901
- PMCID: PMC6756221
- DOI: 10.1038/s41388-019-0750-5
Neurotensin and its receptors mediate neuroendocrine transdifferentiation in prostate cancer
Erratum in
-
Correction: Neurotensin and its receptors mediate neuroendocrine transdifferentiation in prostate cancer.Oncogene. 2019 Jun;38(24):4885. doi: 10.1038/s41388-019-0827-1. Oncogene. 2019. PMID: 31048775 Free PMC article.
Abstract
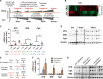
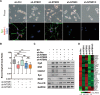
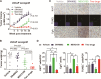
Castration-resistant prostate cancer (CRPC) with neuroendocrine differentiation (NED) is a lethal disease for which effective therapies are urgently needed. The mechanism underlying development of CRPC with NED, however, remains largely uncharacterized. In this study, we explored and characterized the functional role of neurotensin (NTS) in cell line and animal models of CRPC with NED. NTS was acutely induced by androgen deprivation in animal models of prostate cancer (PCa) and activated downstream signaling leading to NED through activation of neurotensin receptor 1 (NTSR1) and neurotensin receptor 3 (NTSR3), but not neurotensin receptor 2 (NTSR2). Our findings also revealed the existence of a CK8+/CK14+ subpopulation in the LNCaP cell line that expresses high levels of both NTSR1 and NTSR3, and displays an enhanced susceptibility to develop neuroendocrine-like phenotypes upon treatment with NTS. More importantly, NTSR1 pathway inhibition prevented the development of NED and castration resistance in vivo. We propose a novel role of NTS in the development of CRPC with NED, and a possible strategy to prevent the onset of NED by targeting the NTS signaling pathway.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

