Detection of axonal degeneration in a mouse model of Huntington's disease: comparison between diffusion tensor imaging and anomalous diffusion metrics
- PMID: 30771034
- PMCID: PMC7837609
- DOI: 10.1007/s10334-019-00742-6
Detection of axonal degeneration in a mouse model of Huntington's disease: comparison between diffusion tensor imaging and anomalous diffusion metrics
Abstract
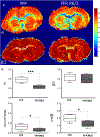
Objective: The goal of this work is to study the changes in white matter integrity in R6/2, a well-established animal model of Huntington's disease (HD) that are captured by ex vivo diffusion imaging (DTI) using a high field MRI (17.6 T).
Materials and methods: DTI and continuous time random walk (CTRW) models were used to fit changes in the diffusion-weighted signal intensity in the corpus callosum of controls and in R6/2 mice.
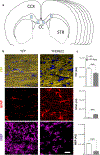
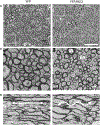
Results: A significant 13% decrease in fractional anisotropy, a 7% increase in axial diffusion, and a 33% increase in radial diffusion were observed between R6/2 and control mice. No change was observed in the CTRW beta parameter, but a significant decrease in the alpha parameter (- 21%) was measured. Histological analysis of the corpus callosum showed a decrease in axonal organization, myelin alterations, and astrogliosis. Electron microscopy studies demonstrated ultrastructural changes in degenerating axons, such as an increase in tortuosity in the R6/2 mice.
Conclusions: DTI and CTRW diffusion models display quantitative changes associated with the microstructural alterations observed in the corpus callosum of the R6/2 mice. The observed increase in the diffusivity and decrease in the alpha CTRW parameter providing support for the use of these diffusion models for non-invasive detection of white matter alterations in HD.
Keywords: Anomalous diffusion; Diffusion tensor imaging; Huntington disease; Magnetic resonance imaging; Mice.
Conflict of interest statement
Figures

References
-
- Harper PS (1992) The epidemiology of Huntington’s disease. Hum Genet 89(4):365–376 - PubMed
-
- Pringsheim T, Wiltshire K, Day L, Dykeman J, Steeves T, Jette N (2012) The incidence and prevalence of Huntington’s disease: a systematic review and meta-analysis. Mov Disord 27(9):1083–1091 - PubMed
-
- Walker FO (2007) Huntington’s disease. Lancet 369(9557):218–228 - PubMed
-
- Perez-Navarro E, Canals JM, Gines S, Alberch J (2006) Cellular and molecular mechanisms involved in the selective vulnerability of striatal projection neurons in Huntington’s disease. Histol Histopathol 21(11):1217–1232 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

