Safety and Efficacy of Belimumab Plus Standard Therapy for Up to Thirteen Years in Patients With Systemic Lupus Erythematosus
- PMID: 30771238
- PMCID: PMC6617785
- DOI: 10.1002/art.40861
Safety and Efficacy of Belimumab Plus Standard Therapy for Up to Thirteen Years in Patients With Systemic Lupus Erythematosus
Abstract
Objective: To investigate the long-term safety and efficacy of intravenous (IV) belimumab plus standard of care (SOC) therapy for systemic lupus erythematosus (SLE) in patients with active, autoantibody-positive SLE.
Methods: The study was designed as a multicenter, open-label, continuation study of IV belimumab given every 4 weeks in conjunction with SOC therapy in patients with SLE who completed a phase II, double-blind study. Adverse events (AEs) and laboratory data were monitored from the first belimumab dose (in either study) until 24 weeks after the final dose. Efficacy assessments included SLE Responder Index (SRI) and flare index scores (each assessed at 16-week intervals) and glucocorticoid use (assessed at 4-week intervals).
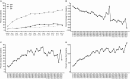
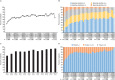
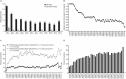
Results: Of the 476 patients in the parent study, 298 (62.6%) entered the continuation study, of whom 96 (32.2%) remained in the study. Patients received belimumab for up to 13 years (median duration of exposure 3,334.0 days [range 260-4,332 days], total belimumab exposure 2,294 patient-years, median number of infusions 115.5 [range 7-155]). The percentage of patients with AEs each year remained stable or decreased. Normal serum IgG levels were maintained in the majority of patients over the study, and the rate of infections remained stable. The percentage of patients who achieved an SRI response increased from 32.8% (year 1) to 75.6% of those remaining on treatment at year 12. The glucocorticoid dose was decreased in patients who had been receiving >7.5 mg/day at baseline.
Conclusion: This study is the longest to date to assess belimumab treatment in patients with SLE in clinical trials. Belimumab was well tolerated with no new safety concerns, and efficacy was maintained in patients who continued the study. For patients who initially exhibited a satisfactory response to belimumab, the treatment continues to be well tolerated and provides long-term disease control.
© 2019 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of Rheumatology.
Figures

References
-
- European Medicines Agency . Benlysta EPAR. 2016. URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Summary_for_th....
-
- Benlysta (belimumab) prescribing information. Rockville (MD): Human Genome Sciences; 2018. URL: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Presc....
-
- GlaxoSmithKline . GSK receives approval for benlysta in Japan for the treatment of systemic lupus erythematosus. URL: https://www.gsk.com/en-gb/media/press-releases/gsk-receives-approval-for....

