Coculture with monocytes/macrophages modulates osteogenic differentiation of adipose-derived mesenchymal stromal cells on poly(lactic-co-glycolic) acid/polycaprolactone scaffolds
- PMID: 30771241
- PMCID: PMC6594112
- DOI: 10.1002/term.2826
Coculture with monocytes/macrophages modulates osteogenic differentiation of adipose-derived mesenchymal stromal cells on poly(lactic-co-glycolic) acid/polycaprolactone scaffolds
Abstract
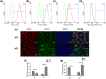
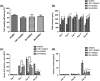
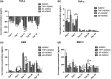
The effects of immune cells, in particular macrophages, on the behaviour of mesenchymal stromal cells (MSCs) have recently gained much attention for MSCs-based tissue-engineered constructs. This study aimed to evaluate the effect of monocytes/macrophages on the osteogenic differentiation of adipose-derived mesenchymal stromal cells (ADMSCs) in three-dimensional (3D) cocultures. For this, we cocultured THP-1 monocytes, M1 macrophages, or M2 macrophages with ADMSCs on 3D poly(lactic-co-glycolic) acid (PLGA)/polycaprolactone (PCL) scaffolds using osteogenic medium for up to 42 days. We found that osteogenic differentiation of ADMSCs was inhibited by monocytes and both macrophage subtypes in 3D scaffolds. Furthermore, coculture of monocytes/macrophages with ADMSCs resulted in downregulated secretion of oncostatin M (OSM) and bone morphogenetic protein 2 (BMP-2) and inhibited expression of osteogenic markers alkaline phosphatase (ALP), bone sialoprotein (BSP), and runt-related transcription factor 2 (RUNX2). Compared with both macrophage subtypes, monocytes inhibited osteogenic differentiation of ADMSCs more significantly. These data suggest that the mutual interactions between monocytes/macrophages and ADMSCs negatively affect MSC osteogenic differentiation and thus possibly bone healing capacity, which highlights the importance of the micro-environment in influencing cell-based constructs to treat bone defects and the potential to improve their performance by resolving the inflammation ahead of treatment.
Keywords: 3D; adipose-derived mesenchymal stromal cells; coculture; macrophages; monocytes; osteogenic differentiation.
© 2019 The Authors. Journal of Tissue Engineering and Regenerative Medicine published by John Wiley & Sons, Ltd.
Conflict of interest statement
The authors have declared that there is no conflict of interest.
Figures
Similar articles
-
Effect of monocytes/macrophages on the osteogenic differentiation of adipose-derived mesenchymal stromal cells in 3D co-culture spheroids.Tissue Cell. 2017 Aug;49(4):461-469. doi: 10.1016/j.tice.2017.06.002. Epub 2017 Jun 15. Tissue Cell. 2017. PMID: 28684045
-
3D-printed nano-hydroxyapatite/poly(lactic-co-glycolic acid) scaffolds with adipose-derived mesenchymal stem cells enhance bone regeneration in rat model of bone defects.J Biomater Appl. 2025 Aug;40(2):284-296. doi: 10.1177/08853282251332050. Epub 2025 Apr 3. J Biomater Appl. 2025. PMID: 40179420
-
Macrophage type modulates osteogenic differentiation of adipose tissue MSCs.Cell Tissue Res. 2017 Aug;369(2):273-286. doi: 10.1007/s00441-017-2598-8. Epub 2017 Mar 30. Cell Tissue Res. 2017. PMID: 28361303 Free PMC article.
-
Coculture of mesenchymal stem cells and macrophage: A narrative review.J Pharmacol Exp Ther. 2025 Apr;392(4):103531. doi: 10.1016/j.jpet.2025.103531. Epub 2025 Mar 5. J Pharmacol Exp Ther. 2025. PMID: 40154096 Review.
-
Mesenchymal stem cells influence monocyte/macrophage phenotype: Regulatory mode and potential clinical applications.Biomed Pharmacother. 2023 Sep;165:115042. doi: 10.1016/j.biopha.2023.115042. Epub 2023 Jun 26. Biomed Pharmacother. 2023. PMID: 37379639 Review.
Cited by
-
Mesenchymal Stem Cell-Macrophage Crosstalk and Maintenance of Inflammatory Microenvironment Homeostasis.Front Cell Dev Biol. 2021 Jun 25;9:681171. doi: 10.3389/fcell.2021.681171. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34249933 Free PMC article. Review.
-
3D Organoids for Regenerative Endodontics.Biomolecules. 2023 May 28;13(6):900. doi: 10.3390/biom13060900. Biomolecules. 2023. PMID: 37371480 Free PMC article. Review.
-
Modeling Immunity In Vitro: Slices, Chips, and Engineered Tissues.Annu Rev Biomed Eng. 2021 Jul 13;23:461-491. doi: 10.1146/annurev-bioeng-082420-124920. Epub 2021 Apr 19. Annu Rev Biomed Eng. 2021. PMID: 33872520 Free PMC article.
-
Characteristics and regulation of mesenchymal stem cell plasticity by the microenvironment - specific factors involved in the regulation of MSC plasticity.Genes Dis. 2020 Oct 27;9(2):296-309. doi: 10.1016/j.gendis.2020.10.006. eCollection 2022 Mar. Genes Dis. 2020. PMID: 35224147 Free PMC article. Review.
-
CXCL chemokines-mediated communication between macrophages and BMSCs on titanium surface promotes osteogenesis via the actin cytoskeleton pathway.Mater Today Bio. 2023 Oct 5;23:100816. doi: 10.1016/j.mtbio.2023.100816. eCollection 2023 Dec. Mater Today Bio. 2023. PMID: 37859997 Free PMC article.
References
-
- American Type Culture Collection (2018). Cells and microorganisms 2016 [28 July 2018]. Available from: https://www.lgcstandards‐atcc.org/en/Products/Cells_and_Microorganisms.aspx.
-
- Astori, G. , Amati, E. , Bambi, F. , Bernardi, M. , Chieregato, K. , Schafer, R. , … Rodeghiero, F. (2016). Platelet lysate as a substitute for animal serum for the ex‐vivo expansion of mesenchymal stem/stromal cells: Present and future. Stem Cell Research & Therapy, 7(1), 93 10.1186/s13287-016-0352-x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

