Optimization of a multivalent peptide vaccine for nicotine addiction
- PMID: 30772068
- PMCID: PMC6417836
- DOI: 10.1016/j.vaccine.2019.02.003
Optimization of a multivalent peptide vaccine for nicotine addiction
Abstract
We have been optimizing the design of a conjugate vaccine for nicotine addiction that employs a peptide-based hapten carrier. This peptide, which is produced by solid-phase protein synthesis, contains B cell and T cell epitope domains and eliminates the non-relevant, but highly immunogenic sequences in microbial carriers. In this report, the amino acid sequences in the T cell domain were optimized for improved vaccine activity and multivalent formulations containing structurally distinct haptens were tested for the induction of additive antibody responses. Trivalent vaccines produced antibody concentrations in mice that were 100 times greater than the amount of nicotine measured in smokers, and significantly reduced acute nicotine toxicity in rats. Two additional features were explored that distinguish the peptide from traditional recombinant carriers. The first is the minimal induction of an anti-carrier response, which can suppress nicotine vaccine activity. The second employs solid-phase synthesis to manufacture haptenated peptide. This approach obviates conventional conjugation chemistries and streamlines production of a more potent vaccine antigen.
Keywords: Addiction; Antibody response; Hapten; Nicotine vaccine; Solid-phase protein synthesis.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interest
All authors are employees of TRIA Bioscience Corp.
Figures
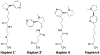


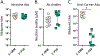





Similar articles
-
Enhancing the Immune Response of a Nicotine Vaccine with Synthetic Small "Non-Natural" Peptides.Molecules. 2020 Mar 12;25(6):1290. doi: 10.3390/molecules25061290. Molecules. 2020. PMID: 32178357 Free PMC article.
-
Novel Anti-Nicotine Vaccine Using a Trimeric Coiled-Coil Hapten Carrier.PLoS One. 2014 Dec 10;9(12):e114366. doi: 10.1371/journal.pone.0114366. eCollection 2014. PLoS One. 2014. PMID: 25494044 Free PMC article.
-
Prescreening of Nicotine Hapten Linkers in Vitro To Select Hapten-Conjugate Vaccine Candidates for Pharmacokinetic Evaluation in Vivo.ACS Comb Sci. 2017 May 8;19(5):286-298. doi: 10.1021/acscombsci.6b00179. Epub 2017 Apr 17. ACS Comb Sci. 2017. PMID: 28383252 Free PMC article.
-
Conjugate Vaccine Immunotherapy for Substance Use Disorder.Pharmacol Rev. 2017 Jul;69(3):298-315. doi: 10.1124/pr.117.013904. Pharmacol Rev. 2017. PMID: 28634286 Free PMC article. Review.
-
New directions in nicotine vaccine design and use.Adv Pharmacol. 2014;69:553-80. doi: 10.1016/B978-0-12-420118-7.00014-7. Adv Pharmacol. 2014. PMID: 24484987 Free PMC article. Review.
Cited by
-
Assessing the immunogenicity and toxicity of the AFPL1-conjugate nicotine vaccine using heterologous and homologous vaccination routes.PLoS One. 2019 Aug 23;14(8):e0221708. doi: 10.1371/journal.pone.0221708. eCollection 2019. PLoS One. 2019. PMID: 31442285 Free PMC article.
-
Diphtheria Toxoid-Derived T-Helper Epitope and α-galactosylceramide Synergistically Enhance the Immunogenicity of Glycopeptide Antigen.ACS Pharmacol Transl Sci. 2024 Nov 4;7(12):3889-3901. doi: 10.1021/acsptsci.4c00437. eCollection 2024 Dec 13. ACS Pharmacol Transl Sci. 2024. PMID: 39698257
-
Epitope-targeting platform for broadly protective influenza vaccines.PLoS One. 2021 May 27;16(5):e0252170. doi: 10.1371/journal.pone.0252170. eCollection 2021. PLoS One. 2021. PMID: 34043704 Free PMC article.
-
Epitope targeting with self-assembled peptide vaccines.NPJ Vaccines. 2019 Jul 19;4:30. doi: 10.1038/s41541-019-0125-5. eCollection 2019. NPJ Vaccines. 2019. PMID: 31341647 Free PMC article.
-
Enhancing the Immune Response of a Nicotine Vaccine with Synthetic Small "Non-Natural" Peptides.Molecules. 2020 Mar 12;25(6):1290. doi: 10.3390/molecules25061290. Molecules. 2020. PMID: 32178357 Free PMC article.
References
-
- NIDA. Media Guide. National Institute on Drug Abuse website; https://www.drugabuse.gov/publications/media-guide. July 2, 2018. Accessed November 12, 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

