Relaxin and fibrosis: Emerging targets, challenges, and future directions
- PMID: 30772373
- PMCID: PMC6475456
- DOI: 10.1016/j.mce.2019.02.005
Relaxin and fibrosis: Emerging targets, challenges, and future directions
Abstract
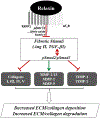
The peptide hormone relaxin is well-known for its anti-fibrotic actions in several organs, particularly from numerous studies conducted in animals. Acting through its cognate G protein-coupled receptor, relaxin family peptide receptor 1 (RXFP1), serelaxin (recombinant human relaxin) has been shown to consistently inhibit the excessive extracellular matrix production (fibrosis) that results from the aberrant wound-healing response to tissue injury and/or chronic inflammation, and at multiple levels. Furthermore, it can reduce established scarring by promoting the degradation of aberrant extracellular matrix components. Following on from the review that describes the mechanisms and signaling pathways associated with the extracellular matrix remodeling effects of serelaxin (Ng et al., 2019), this review focuses on newly identified tissue targets of serelaxin therapy in fibrosis, and the limitations associated with (se)relaxin research.
Keywords: Challenges and future directions; Emerging targets; Extracellular matrix; Fibrosis; RXFP1; Relaxin.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest statement: The authors report no conflict of interest.
Figures



References
-
- Aitken KJ, Bagli DJ, 2009. The bladder extracellular matrix. Part I: architecture, development and disease. Nat. Rev. Urol 6, 596–611. - PubMed
-
- Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ, 2013. Relaxin family peptides and their receptors. Physiol. Rev 93, 405–480. - PubMed
-
- Boyle PM, Hakim JB, Zahid S, Franceschi WH, Murphy MJ, Prakosa A, Aronis KN, Zghaib T, Balouch M, Ipek EG, Chrispin J, Berger RD, Ashikaga H, Marine JE, Calkins H, Nazarian S, Spragg DD, Trayanova NA, 2018. The fibrotic substrate in persistent atrial fibrillation patients: comparison between predictions from computational modeling and measurements from focal impulse and rotor mapping. Front. Physiol 9, 1151. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

