Design and baseline characteristics of a low-income urban cohort of children with asthma: The Asthma Action at Erie Trial
- PMID: 30772471
- PMCID: PMC6541387
- DOI: 10.1016/j.cct.2019.02.006
Design and baseline characteristics of a low-income urban cohort of children with asthma: The Asthma Action at Erie Trial
Abstract
Objective: To describe the methodology of a randomized controlled trial comparing the efficacy of integrated asthma community health workers (CHW) and a certified asthma educator (AE-C) to improve asthma outcomes in low-income minority children in Chicago.
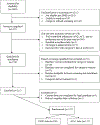
Methods: Child/caregiver dyads were randomized to CHW home visits or education in the clinic from an AE-C. Intervention was delivered in the first year after enrollment. Data collection occured at baseline, 6-, 12-, 18, and 24-months. The co-primary outcomes included asthma control using the Asthma Control Test/childhood Asthma Control Test (ACT/cACT) and activity limitation over the past 14 days.
Results: A total of 223 participants ages 5-16 years were randomized. The majority of children were in the 5-11 year old range (78.9%). Most caregivers (96.9%) and 44% of children were female. Approximately 85% of caregivers and children reported Hispanic ethnicity and 62.3% reported a household income of ≤ $59,000. Over half (55.7%) had uncontrolled asthma as measured by ACT/cACT; 13.9% had a normal ACT/cACT score but were uncontrolled using the Asthma Control Questionnaire and 20.2% were controlled on both measures but had received oral steroids in the past year for asthma.
Conclusion: The Asthma Action at Erie Trial successfully recruited a largely Hispanic cohort of children with uncontrolled or high-risk asthma to study the differential effects of clinic-based AE-C and home-based CHW interventions. Strengths of the trial include its comparative effectivness design that integrates interventionists and intervention delivery into a clinical setting. Categorizing asthma control in community settings for research purposes presents unique challenges.
Clinical trial registration: University of Illinois at Chicago Protocol Record R01HL123797, Asthma Action at Erie TrialClinicalTrials.gov Identifier: NCT02481986 "ClinicalTrials.gov Registration" register@clinicaltrials.gov.
Keywords: Adherence; Asthma; Community health workers; Disparities; Health outcomes; Pediatrics.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
References
-
- CDC. 2016 National Health Interview Survey (NHIS). 2016.
-
- Nurmagambetov T KR, Garbe P, the economic burgen of asthma in the United States 2008-2013. Ann Am Thorac Soc, 2018. 3(15): p. 348–56. - PubMed
-
- Akinbami LJ, et al., Status of childhood asthma in the United States, 1980-2007. Pediatrics, 2009. 123 Suppl 3: p. S131–45. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous